Panthenol Citrate Biomaterials Accelerate Wound Healing and Restore Tissue Integrity
- PMID: 37327023
- PMCID: PMC11468745
- DOI: 10.1002/adhm.202301683
Panthenol Citrate Biomaterials Accelerate Wound Healing and Restore Tissue Integrity
Abstract
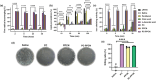
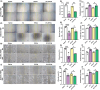
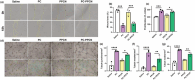
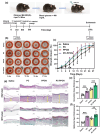
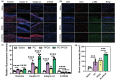
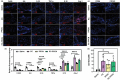
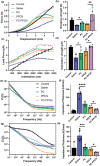
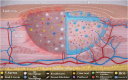
Impaired wound healing is a common complication for diabetic patients and effective diabetic wound management remains a clinical challenge. Furthermore, a significant problem that contributes to patient morbidity is the suboptimal quality of healed skin, which often leads to reoccurring chronic skin wounds. Herein, a novel compound and biomaterial building block, panthenol citrate (PC), is developed. It has interesting fluorescence and absorbance properties, and it is shown that PC can be used in soluble form as a wash solution and as a hydrogel dressing to address impaired wound healing in diabetes. PC exhibits antioxidant, antibacterial, anti-inflammatory, and pro-angiogenic properties, and promotes keratinocyte and dermal fibroblast migration and proliferation. When applied in a splinted excisional wound diabetic rodent model, PC improves re-epithelialization, granulation tissue formation, and neovascularization. It also reduces inflammation and oxidative stress in the wound environment. Most importantly, it improves the regenerated tissue quality with enhanced mechanical strength and electrical properties. Therefore, PC could potentially improve wound care management for diabetic patients and play a beneficial role in other tissue regeneration applications.
Keywords: diabetic wound healing; panthenol citrate; provitamin B5; regenerative engineering and medicine.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
G.A.A. is an inventor on patents that disclose PC and PC‐PPCN. The remaining authors declare no conflict of interest.
Figures

Similar articles
-
Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair.Theranostics. 2020 Mar 31;10(11):4929-4943. doi: 10.7150/thno.41839. eCollection 2020. Theranostics. 2020. PMID: 32308759 Free PMC article.
-
Keratin Scaffolds Containing Casomorphin Stimulate Macrophage Infiltration and Accelerate Full-Thickness Cutaneous Wound Healing in Diabetic Mice.Molecules. 2021 Apr 27;26(9):2554. doi: 10.3390/molecules26092554. Molecules. 2021. PMID: 33925737 Free PMC article.
-
Targeted Nrf2 activation therapy with RTA 408 enhances regenerative capacity of diabetic wounds.Diabetes Res Clin Pract. 2018 May;139:11-23. doi: 10.1016/j.diabres.2018.02.021. Epub 2018 Feb 21. Diabetes Res Clin Pract. 2018. PMID: 29476889
-
Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside.Acta Biomater. 2020 Feb;103:24-51. doi: 10.1016/j.actbio.2019.11.050. Epub 2019 Dec 2. Acta Biomater. 2020. PMID: 31805409 Review.
-
Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing.Biomolecules. 2022 Dec 12;12(12):1852. doi: 10.3390/biom12121852. Biomolecules. 2022. PMID: 36551280 Free PMC article. Review.
Cited by
-
Cell-free biodegradable electroactive scaffold for urinary bladder tissue regeneration.Nat Commun. 2025 Jan 2;16(1):11. doi: 10.1038/s41467-024-55401-9. Nat Commun. 2025. PMID: 39746994 Free PMC article.
-
Clinical profiling of skin microbiome and metabolome during re-epithelialization.Sci Rep. 2025 Jul 1;15(1):22282. doi: 10.1038/s41598-025-07547-9. Sci Rep. 2025. PMID: 40596567 Free PMC article.
-
Exo-hydrogel therapy: a revolutionary approach to managing diabetic complications.J Nanobiotechnology. 2025 Aug 11;23(1):558. doi: 10.1186/s12951-025-03621-6. J Nanobiotechnology. 2025. PMID: 40790200 Free PMC article. Review.
-
Bioresorbable Materials for Wound Management.Biomimetics (Basel). 2025 Feb 12;10(2):108. doi: 10.3390/biomimetics10020108. Biomimetics (Basel). 2025. PMID: 39997131 Free PMC article. Review.
-
Prebiotic- and Panthenol-Containing Multipurpose Healing Dermocosmetics Post-Cryotherapy for Actinic Keratoses: Results of a Randomized Controlled Trial.Dermatol Pract Concept. 2025 Jan 30;15(1):4905. doi: 10.5826/dpc.1501a4905. Dermatol Pract Concept. 2025. PMID: 40117599 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

