Multicenter phase 2 study of romidepsin plus lenalidomide for previously untreated peripheral T-cell lymphoma
- PMID: 37327113
- PMCID: PMC10561000
- DOI: 10.1182/bloodadvances.2023009767
Multicenter phase 2 study of romidepsin plus lenalidomide for previously untreated peripheral T-cell lymphoma
Abstract
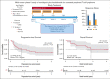
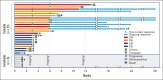
Peripheral T-cell lymphomas (PTCLs) are associated with poor prognosis when treated with cytotoxic chemotherapy. We report the findings of a phase 2 study evaluating a chemotherapy-free combination of romidepsin plus lenalidomide as initial treatment for patients with PTCL who were aged >60 years or noncandidates for chemotherapy. Treatment was initiated with romidepsin 10 mg/m2 IV on days 1, 8, and 15 and lenalidomide 25 mg taken orally from days 1 to 21 of 28-day cycle for up to 1 year. The primary objective was overall response rate (ORR). Secondary objectives included safety and survival. The study enrolled 29 patients with a median age of 75 years, including 16 (55%) angioimmunoblastic T-cell lymphoma (AITL), 10 (34%) PTCL- not otherwise specified, 2 ATLL, and 1 EATL. Grade 3 to 4 hematologic toxicities included neutropenia (45%), thrombocytopenia (34%), and anemia (28%). Grade 3 to 4 nonhematologic toxicities included hyponatremia (45%), hypertension (38%), hypoalbuminemia (24%), fatigue (17%), hyperglycemia (14%), hypokalemia (14%), dehydration (10%), and infection (10%). At median follow-up of 15.7 months, 23 patients were evaluable and received a median treatment of 6 cycles. The ORR was 65.2% with complete response (CR) at 26.1%, including 78.6% ORR and 35.7% CR for AITL. Median duration of response was 10.7 months, with 27.1 months for patients achieving CR. The estimated 2-year progression-free survival was 31.5%, and 2-year overall survival was 49.5%. This study provides the first demonstration that the biologic combination of romidepsin and lenalidomide is feasible and effective as initial therapy for PTCL and warrants further evaluation. This trial was registered at www.clinicaltrials.gov as #NCT02232516.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.R. has received research support, provided consultancy, and served on the advisory board meetings for Bristol Myers Squibb (BMS)/Celgene, AstraZeneca, Genentech, Daiichi Sankyo, Seattle Genetics, Secura Bio and Kite Pharma. B.P. is currently employed by Hutchmed. J.N.W. has received research support from Merck. L.I.G. has served on the data and safety monitoring board for Janssen; served on the advisory board for Kite Pharma and BMS; and provided consultancy for Ono Pharmaceutical. R.K. has served on the advisory boards for BMS, Gilead Sciences/Kite Pharma, Janssen, Karyopharm, Pharmacyclics, MorphoSys, Epizyme, Genentech/Roche, EUSA, Calithera, and Janssen; has received research support from BMS, Takeda, BeiGene, Gilead Sciences/Kite Pharma; and has received honoraria from AstraZeneca, BeiGene, and MorphoSys. B.P. has received research support and honoraria from Seattle Genetics and Takeda, and has received research support from Celgene. The remaining authors declare no competing financial interests.
Figures


References
-
- Vose J, Armitage J, Weisenburger D, International T-Cell Lymphoma Project International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. - PubMed
-
- Reimer P, Rüdiger T, Geissinger E, et al. Autologous stem-cell transplantation as first-line therapy in peripheral t-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27(1):106–113. - PubMed
-
- Simon A, Peoch M, Casassus P, et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol. 2010;151(2):159–166. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

