Hexokinase dissociation from mitochondria promotes oligomerization of VDAC that facilitates NLRP3 inflammasome assembly and activation
- PMID: 37327321
- PMCID: PMC10360408
- DOI: 10.1126/sciimmunol.ade7652
Hexokinase dissociation from mitochondria promotes oligomerization of VDAC that facilitates NLRP3 inflammasome assembly and activation
Abstract
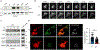
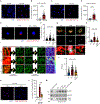
NLRP3 inflammasome activation is a highly regulated process for controlling secretion of the potent inflammatory cytokines IL-1β and IL-18 that are essential during bacterial infection, sterile inflammation, and disease, including colitis, diabetes, Alzheimer's disease, and atherosclerosis. Diverse stimuli activate the NLRP3 inflammasome, and unifying upstream signals has been challenging to identify. Here, we report that a common upstream step in NLRP3 inflammasome activation is the dissociation of the glycolytic enzyme hexokinase 2 from the voltage-dependent anion channel (VDAC) in the outer membrane of mitochondria. Hexokinase 2 dissociation from VDAC triggers activation of inositol triphosphate receptors, leading to release of calcium from the ER, which is taken up by mitochondria. This influx of calcium into mitochondria leads to oligomerization of VDAC, which is known to form a macromolecule-sized pore in the outer membranes of mitochondria that allows proteins and mitochondrial DNA (mtDNA), often associated with apoptosis and inflammation, respectively, to exit the mitochondria. We observe that VDAC oligomers aggregate with NLRP3 during initial assembly of the multiprotein oligomeric NLRP3 inflammasome complex. We also find that mtDNA is necessary for NLRP3 association with VDAC oligomers. These data, together with other recent work, help to paint a more complete picture of the pathway leading to NLRP3 inflammasome activation.
Conflict of interest statement
Figures
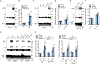

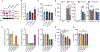

References
-
- Tzeng T-C, Golenbock D, NLRP3 inflammasome activation in Alzheimer’s disease (INC9P.446). J. Immunol 192, 188.185–188.185 (2014).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

