Enantioselective synthesis of α,α-diarylketones by sequential visible light photoactivation and phosphoric acid catalysis
- PMID: 37327329
- PMCID: PMC10275591
- DOI: 10.1126/sciadv.adg7754
Enantioselective synthesis of α,α-diarylketones by sequential visible light photoactivation and phosphoric acid catalysis
Abstract
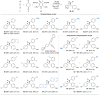
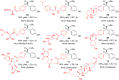
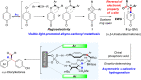
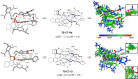
Chiral ketones and their derivatives are useful synthetic intermediates for the synthesis of biologically active natural products and medicinally relevant molecules. Nevertheless, general and broadly applicable methods for enantioenriched acyclic α,α-disubstituted ketones, especially α,α-diarylketones, remain largely underdeveloped, owing to the easy racemization. Here, we report a visible light photoactivation and phosphoric acid-catalyzed alkyne-carbonyl metathesis/transfer hydrogenation one-pot reaction using arylalkyne, benzoquinone, and Hantzsch ester for the expeditious synthesis of α,α-diarylketones with excellent yields and enantioselectivities. In the reaction, three chemical bonds, including C═O, C─C, and C─H, are formed, providing a de novo synthesis reaction for chiral α,α-diarylketones. Moreover, this protocol provides a convenient and practical method to synthesize or modify complex bioactive molecules, including efficient routes to florylpicoxamid and BRL-15572 analogs. Computational mechanistic studies revealed that C-H/π interactions, π-π interaction, and the substituents of Hantzsch ester all play crucial roles in the stereocontrol of the reaction.
Figures




Similar articles
-
Benzothiazoline: versatile hydrogen donor for organocatalytic transfer hydrogenation.Acc Chem Res. 2015 Feb 17;48(2):388-98. doi: 10.1021/ar500414x. Epub 2015 Jan 22. Acc Chem Res. 2015. PMID: 25611073 Review.
-
Chiral phosphoric acid catalyzed highly enantioselective Friedel-Crafts alkylation reaction of C3-substituted indoles to β,γ-unsaturated α-ketimino esters.Org Lett. 2015 Feb 6;17(3):540-3. doi: 10.1021/ol5035222. Epub 2015 Jan 16. Org Lett. 2015. PMID: 25594307
-
DFT study of the mechanism and origin of enantioselectivity in chiral BINOL-phosphoric acid catalyzed transfer hydrogenation of ketimine and α-imino ester using benzothiazoline.J Org Chem. 2013 Apr 19;78(8):3731-6. doi: 10.1021/jo4002195. Epub 2013 Apr 4. J Org Chem. 2013. PMID: 23521654
-
Enantioselective De Novo Synthesis of α,α-Diaryl Ketones from Alkynes.Angew Chem Int Ed Engl. 2023 Nov 6;62(45):e202310078. doi: 10.1002/anie.202310078. Epub 2023 Sep 28. Angew Chem Int Ed Engl. 2023. PMID: 37724448
-
Diastereo- and enantioselective anti-selective hydrogenation of α-amino-β-keto ester hydrochlorides and related compounds using transition-metal-chiral-bisphosphine catalysts.Chem Rec. 2014 Apr;14(2):235-50. doi: 10.1002/tcr.201300032. Epub 2014 Feb 18. Chem Rec. 2014. PMID: 24550034 Review.
Cited by
-
Asymmetric C2-functionalization of indoles via visible-light-promoted three-component reaction.Mol Divers. 2025 Aug 15. doi: 10.1007/s11030-025-11319-y. Online ahead of print. Mol Divers. 2025. PMID: 40815359
-
Catalytic Asymmetric Construction of α,α-Diaryl Aldehydes via Oxo-Hydroarylation of Terminal Alkynes.Adv Sci (Weinh). 2024 Jun;11(24):e2309645. doi: 10.1002/advs.202309645. Epub 2024 Apr 22. Adv Sci (Weinh). 2024. PMID: 38650176 Free PMC article.
-
Palladium complex supported on the surface of magnetic Fe3O4 nanoparticles: an ecofriendly catalyst for carbonylative Suzuki-coupling reactions.RSC Adv. 2023 Dec 1;13(48):34273-34290. doi: 10.1039/d3ra06533b. eCollection 2023 Nov 16. RSC Adv. 2023. Retraction in: RSC Adv. 2025 May 15;15(21):16266. doi: 10.1039/d5ra90061a. PMID: 38047105 Free PMC article. Retracted.
References
-
- M. Sankalan, R. Deblina, P. Gautam, Overview on the recent strategies for the enantioselective synthesis of 1, 1-diarylalkanes, triarylmethanes and related molecules containing the diarylmethine stereocenter. ChemCatChem 10, 1941–1967 (2018).
-
- M. Belal, Z. Li, X. Lu, G. Yin, Recent advances in the synthesis of 1,1-diarylalkanes by transition-metal catalysis. Sci. Chi. Chem. 64, 513–533 (2021).
-
- S.-Y. Wu, Y.-H. Fu, Q. Zhou, M. Bai, G.-Y. Chen, C.-R. Han, X.-P. Song, Biologically active oligostilbenes from the stems of Vatica mangachapoi and chemotaxonomic significance. Nat. Prod. Res. 33, 2300–2307 (2019). - PubMed
-
- Y.-C. He, C. Peng, X.-F. Xie, M.-H. Chen, X.-N. Li, M.-T. Li, Q.-M. Zhou, L. Guo, L. Xiong, Penchinones A–D, two pairs of cis-trans isomers with rearranged neolignane carbon skeletons from Penthorum chinense. RSC Adv. 5, 76788–76794 (2015).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

