A Plasmodium membrane receptor platform integrates cues for egress and invasion in blood forms and activation of transmission stages
- PMID: 37327340
- PMCID: PMC10275601
- DOI: 10.1126/sciadv.adf2161
A Plasmodium membrane receptor platform integrates cues for egress and invasion in blood forms and activation of transmission stages
Abstract
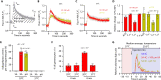
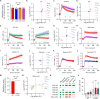
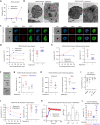
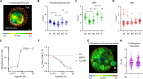
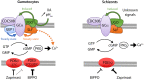
Critical events in the life cycle of malaria-causing parasites depend on cyclic guanosine monophosphate homeostasis by guanylyl cyclases (GCs) and phosphodiesterases, including merozoite egress or invasion of erythrocytes and gametocyte activation. These processes rely on a single GCα, but in the absence of known signaling receptors, how this pathway integrates distinct triggers is unknown. We show that temperature-dependent epistatic interactions between phosphodiesterases counterbalance GCα basal activity preventing gametocyte activation before mosquito blood feed. GCα interacts with two multipass membrane cofactors in schizonts and gametocytes: UGO (unique GC organizer) and SLF (signaling linking factor). While SLF regulates GCα basal activity, UGO is essential for GCα up-regulation in response to natural signals inducing merozoite egress and gametocyte activation. This work identifies a GC membrane receptor platform that senses signals triggering processes specific to an intracellular parasitic lifestyle, including host cell egress and invasion to ensure intraerythrocytic amplification and transmission to mosquitoes.
Figures



References
-
- R. E. Sinden, The cell biology of sexual development in Plasmodium. Parasitology 86, 7–28 (1983). - PubMed
-
- O. Billker, V. Lindo, M. Panico, A. E. Etienne, T. Paxton, A. Dell, M. Rogers, R. E. Sinden, H. R. Morris, Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 392, 289–292 (1998). - PubMed
-
- D. S. Yamamoto, M. Sumitani, M. Hatakeyama, H. Matsuoka, Malaria infectivity of xanthurenic acid-deficient anopheline mosquitoes produced by TALEN-mediated targeted mutagenesis. Transgenic Res. 27, 51–60 (2018). - PubMed
-
- M. Arai, O. Billker, H. R. Morris, M. Panico, M. Delcroix, D. Dixon, S. V. Ley, R. E. Sinden, Both mosquito-derived xanthurenic acid and a host blood-derived factor regulate gametogenesis of Plasmodium in the midgut of the mosquito. Mol. Biochem. Parasitol. 116, 17–24 (2001). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

