Skeletal muscle in amyotrophic lateral sclerosis
- PMID: 37327376
- PMCID: PMC10629757
- DOI: 10.1093/brain/awad202
Skeletal muscle in amyotrophic lateral sclerosis
Abstract
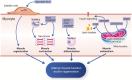
Amyotrophic lateral sclerosis (ALS), the major adult-onset motor neuron disease, has been viewed almost exclusively as a disease of upper and lower motor neurons, with muscle changes interpreted as a consequence of the progressive loss of motor neurons and neuromuscular junctions. This has led to the prevailing view that the involvement of muscle in ALS is only secondary to motor neuron loss. Skeletal muscle and motor neurons reciprocally influence their respective development and constitute a single functional unit. In ALS, multiple studies indicate that skeletal muscle dysfunction might contribute to progressive muscle weakness, as well as to the final demise of neuromuscular junctions and motor neurons. Furthermore, skeletal muscle has been shown to participate in disease pathogenesis of several monogenic diseases closely related to ALS. Here, we move the narrative towards a better appreciation of muscle as a contributor of disease in ALS. We review the various potential roles of skeletal muscle cells in ALS, from passive bystanders to active players in ALS pathophysiology. We also compare ALS to other motor neuron diseases and draw perspectives for future research and treatment.
Keywords: denervation; energy metabolism; mouse models; neuromuscular junction; neurophysiology.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
J.M.S., A.M., S.T.N., C.L., F.S., R.R., M.d.C., S.R., A.C.L. and L.D. report consultancy fees from Cytokinetics.
Figures



References
-
- Brown RH Jr, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162–172. - PubMed
-
- Liu W, Chakkalakal JV. The composition, development, and regeneration of neuromuscular junctions. Curr Top Dev Biol. 2018;126:99–124. - PubMed
-
- Li L, Xiong WC, Mei L. Neuromuscular junction formation, aging, and disorders. Annu Rev Physiol. 2018;80:159–188. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

