The Clock-modulatory Activity of Nobiletin Suppresses Adipogenesis Via Wnt Signaling
- PMID: 37327385
- PMCID: PMC10373950
- DOI: 10.1210/endocr/bqad096
The Clock-modulatory Activity of Nobiletin Suppresses Adipogenesis Via Wnt Signaling
Abstract
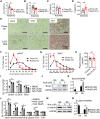
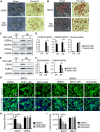
The circadian clock machinery exerts transcriptional control to modulate adipogenesis and its disruption leads to the development of obesity. Here, we report that Nobiletin, a circadian clock amplitude-enhancing molecule, displays antiadipogenic properties via activation of Wnt signaling pathway that is dependent on its clock modulation. Nobiletin augmented clock oscillatory amplitude with period lengthening in the adipogenic mesenchymal precursor cells and preadipocytes, accompanied by an induction of Bmal1 and clock components within the negative feedback arm. Consistent with its clock-modulatory activity, Nobiletin strongly inhibited the lineage commitment and terminal differentiation of adipogenic progenitors. Mechanistically, we show that Nobiletin induced the reactivation of Wnt signaling during adipogenesis via transcriptional up-regulation of key components within this pathway. Furthermore, Nobiletin administration in mice markedly reduced adipocyte hypertrophy, leading to a significant loss of fat mass and reduction of body weight. Last, Nobiletin inhibited the differentiation of primary preadipocytes, and this effect was dependent on a functional clock regulation. Collectively, our findings uncover a novel activity of Nobiletin in suppressing adipocyte development in a clock-dependent manner, implicating its potential application in countering obesity and associated metabolic consequences.
Keywords: Wnt signaling; adipocyte development; adipogenesis; circadian clock; differentiation.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





Update of
-
The clock-modulatory activity of Nobiletin suppresses adipogenesis via Wnt signaling.bioRxiv [Preprint]. 2023 Feb 8:2023.02.07.527587. doi: 10.1101/2023.02.07.527587. bioRxiv. 2023. Update in: Endocrinology. 2023 Jun 26;164(8):bqad096. doi: 10.1210/endocr/bqad096. PMID: 36798247 Free PMC article. Updated. Preprint.
References
-
- Panda S, Antoch MP, Miller BH, et al. . Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307‐320. - PubMed
-
- Stenvers DJ, Scheer F, Schrauwen P, La Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 2019;15(2):75‐89. - PubMed

