Malfunctioned inflammatory response and serotonin metabolism at the microbiota-gut-brain axis drive feather pecking behavior in laying hens
- PMID: 37327743
- PMCID: PMC10404692
- DOI: 10.1016/j.psj.2023.102686
Malfunctioned inflammatory response and serotonin metabolism at the microbiota-gut-brain axis drive feather pecking behavior in laying hens
Abstract
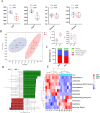
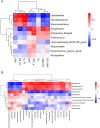
Feather pecking (FP) is a multifactorial abnormal behavior in laying hens where they display harmful pecks in conspecifics. FP has been associated with the altered functioning of the microbiome-gut-brain axis affecting host emotions and social behavior. The altered levels of serotonin (5-HT), a key monoaminergic neurotransmitter at both terminals of the gut-brain axis, affect the development of abnormal behavior, such as FP in laying hens. However, the underlying mechanism involving reciprocal interactions along the microbiota-gut-brain axis, particularly about the metabolism of 5-HT, remains unclear in FP phenotypes. This study examined the microbiota diversity, intestinal microbial metabolites, inflammatory responses, and 5-HT metabolism in divergently selected high (HFP; n = 8) and low (LFP; n = 8) FP hens to investigate the possible interconnections between FP behavior and the examined parameters. The 16S rRNA analysis revealed that compared to LFP birds, the gut microbiota of HFP birds exhibited a decrease in the abundance of phylum Firmicutes and genera Lactobacillus, while an increase in the abundance of phylum Proteobacteria and genera Escherichia Shigella and Desulfovibrio. Furthermore, the intestinal differential metabolites associated with FP phenotypes were mainly enriched in the tryptophan metabolic pathway. HFP birds had higher tryptophan metabolites and possibly a more responsive immune system compared to the LFP birds. This was indirectly supported by altered TNF-α levels in the serum and expression of inflammatory factor in the gut and brain. Moreover, HFP birds had lower serum levels of tryptophan and 5-HT compared to LFP birds, which was consistent with the downregulation of 5-HT metabolism-related genes in the brain of HFP birds. The correlation analysis revealed that genera Lactobacillus and Desulfovibrio were associated with differences in intestinal metabolites, 5-HT metabolism, and inflammatory response between the LFP and HFP birds. In conclusion, differences in the cecal microbiota profile, immune response and 5-HT metabolism drive FP phenotypes, which could be associated with the gut abundance of genera Lactobacillus and Desulfovibrio.
Keywords: 5-HT metabolism; feather pecking; inflammatory response in laying hen; microbiome-gut-brain axis.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Bestman M., Verwer C., Brenninkmeyer C., Willett A., Hinrichsen L.K., Smajlhodzic F., Heerkens J.L.T., Gunnarsson S., Ferrante V. Feather-pecking and injurious pecking in organic laying hens in 107 flocks from eight European countries. Anim. Welfare. 2017;26:355–363.
-
- Bolhuis J.E., Ellen E.D., Van Reenen C.G., De Groot J., Ten Napel J., Koopmanschap R.E., Reilingh G.D.V., Uitdehaag K.A., Kemp B., Rodenburg T.B. Effects of genetic group selection against mortality on behavior and peripheral serotonin in domestic laying hens with trimmed and intact beaks. Physiol. Behav. 2009;97:470–475. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

