Uterus transplantation: from research, through human trials and into the future
- PMID: 37328434
- PMCID: PMC10477946
- DOI: 10.1093/humupd/dmad012
Uterus transplantation: from research, through human trials and into the future
Abstract
Women suffering from absolute uterine factor infertility (AUFI) had no hope of childbearing until clinical feasibility of uterus transplantation (UTx) was documented in 2014 with the birth of a healthy baby. This landmark accomplishment followed extensive foundational work with a wide range of animal species including higher primates. In the present review, we provide a summary of the animal research and describe the results of cases and clinical trials on UTx. Surgical advances for graft removal from live donors and transplantation to recipients are improving, with a recent trend away from laparotomy to robotic approaches, although challenges persist regarding optimum immunosuppressive therapies and tests for graft rejection. Because UTx does not involve transplantation of the Fallopian tubes, IVF is required as part of the UTx process. We provide a unique focus on the intersection between these two processes, with consideration of when oocyte retrieval should be performed, whether, and for whom, preimplantation genetic testing for aneuploidy should be used, whether oocytes or embryos should be frozen and when the first embryo transfer should be performed post-UTx. We also address the utility of an international society UTx (ISUTx) registry for assessing overall UTx success rates, complications, and live births. The long-term health outcomes of all parties involved-the uterus donor (if live donor), the recipient, her partner and any children born from the transplanted graft-are also reviewed. Unlike traditional solid organ transplantation procedures, UTx is not lifesaving, but is life-giving, although as with traditional types of transplantation, costs, and ethical considerations are inevitable. We discuss the likelihood that costs will decrease as efficiency and efficacy improve, and that ethical complexities for and against acceptability of the procedure sharpen the distinctions between genetic, gestational, and social parenthood. As more programs wish to offer the procedure, we suggest a scheme for setting up a UTx program as well as future directions of this rapidly evolving field. In our 2010 review, we described the future of clinical UTx based on development of the procedure in animal models. This Grand Theme Review offers a closing loop to this previous review of more than a decade ago. The clinical feasibility of UTx has now been proved. Advancements include widening the criteria for acceptance of donors and recipients, improving surgery, shortening time to pregnancy, and improving post-UTx management. Together, these improvements catalyze the transition of UTx from experimental into mainstream clinical practice. The procedure will then represent a realistic and accessible alternative to gestational surrogacy for the treatment of AUFI and should become part of the armamentarium of reproductive specialists worldwide.
Keywords: IVF; animal models; assisted reproduction; ethics; human; infertility; surgery; transplantation; uterine factor infertility; uterus.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
No author reports any conflict of interest regarding the contents of this manuscript.
Figures
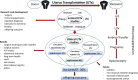
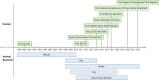

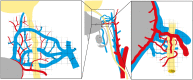
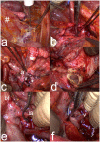


References
-
- Akouri R, Maalouf G, Abboud J, Nakad T, Bedran F, Hajj P, Beaini C, Cricu L, Aftimos G, Hajj C. et al. First live birth after uterus transplantation in the Middle East. MEFS J 2020;25:30–35.
-
- Arantes RM, Nacif LS, Pinheiro RS, Rocha-Santos V, de Martino RB, Waisberg DR, Pantanali CAR, Fortunato A, Lima MR, Ducatti L. et al. Novel technique in a sheep model of uterine transplantation. Transplant Proc 2020;52:1399–1401. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

