Evaluation of inactivation of bovine coronavirus by low-level radiofrequency irradiation
- PMID: 37328590
- PMCID: PMC10275941
- DOI: 10.1038/s41598-023-36887-7
Evaluation of inactivation of bovine coronavirus by low-level radiofrequency irradiation
Abstract
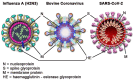
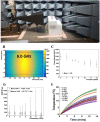
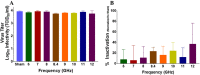
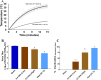
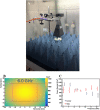
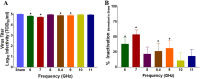
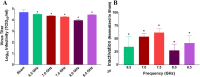
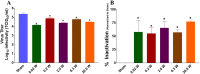
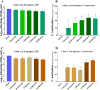
Inactivation of influenza A virus by radiofrequency (RF) energy exposure at levels near Institute of Electrical and Electronics Engineers (IEEE) safety thresholds has been reported. The authors hypothesized that this inactivation was through a structure-resonant energy transfer mechanism. If this hypothesis is confirmed, such a technology could be used to prevent transmission of virus in occupied public spaces where RF irradiation of surfaces could be performed at scale. The present study aims to both replicate and expand the previous work by investigating the neutralization of bovine coronavirus (BCoV), a surrogate of SARS-CoV-2, by RF radiation in 6-12 GHz range. Results showed an appreciable reduction in BCoV infectivity (up to 77%) due to RF exposure to certain frequencies, but failed to generate enough reduction to be considered clinically significant.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Khan MH, Yadav H. Sanitization during and after COVID-19 pandemic: A short review. Trans. Indian Natl. Acad. Eng. 2020;5:617–627. doi: 10.1007/s41403-020-00177-9. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

