YAP/STAT3 inhibited CD8+ T cells activity in the breast cancer immune microenvironment by inducing M2 polarization of tumor-associated macrophages
- PMID: 37329188
- PMCID: PMC10469732
- DOI: 10.1002/cam4.6242
YAP/STAT3 inhibited CD8+ T cells activity in the breast cancer immune microenvironment by inducing M2 polarization of tumor-associated macrophages
Abstract
Background: Breast cancer (BC) is the leading cause of cancer-related death among women. One of the hallmarks of cancer is sustained angiogenesis. YAP/STAT3 may promote angiogenesis and driving BC progression. This study aimed to investigate how YAP/STAT3 affects the immune microenvironment in BC and understand the underlying mechanism.
Methods: To establish a tumor-associated macrophages (TAMs) model, macrophages were cultured in the 4T1 cell culture medium. A BC mouse model was created by injecting 4T1 cells. The expression of YAP, STAT3, p-STAT3, VEGF, VEGFR-2, and PD-L1 was analyzed using immunofluorescence, western blotting, and quantitative real-time PCR. Flow cytometry was used to identify M1 and M2 macrophages, CD4+ T, CD8+ T, and Treg cells. Levels of iNOS, IL-12, IL-10, TGF-β, Arg-1, and CCL-22 were measured using enzyme-linked immunosorbent assay. Co-IP was used to verify whether YAP binds to STAT3. Hematoxylin-eosin staining was used to observe tumor morphology. Cell counting kit-8 was selected to detect T-cell proliferation.
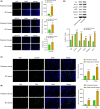
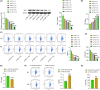
Results: YAP, STAT3, P-STAT3, VEGF, VEGFR-2, and PD-L1 were highly expressed in BC tissues. The M2/M1 macrophages ratio increased in the TAMs group compared with the control group. Inhibiting of YAP and STAT3 decreased the M2/M1 macrophages ratio. YAP was found to bind with STAT3. T-cell proliferation was enhanced after YAP inhibition, and overexpression of STAT3 reversed the regulation of YAP on T-cell proliferation. In animal studies, inhibiting YAP inhibited tumor weight and volume development. After YAP inhibition, inflammatory infiltration, M2/M1 macrophage ratio, and Treg cell ratio declined, while CD8+ and CD4+ T-cell ratio increased.
Conclusion: In conclusion, this study suggested inhibition of YAP/STAT3 reversed M2 polarization of TAMs and suppressed CD8+ T-cell activity in the BC immune microenvironment. These findings open up new avenues for the development of innovative therapies in the treatment of BC.
Keywords: CD8+ T; TAMs; YAP/STAT3; breast cancer.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures



Similar articles
-
Taraxacum mongolicum extract inhibited malignant phenotype of triple-negative breast cancer cells in tumor-associated macrophages microenvironment through suppressing IL-10 / STAT3 / PD-L1 signaling pathways.J Ethnopharmacol. 2021 Jun 28;274:113978. doi: 10.1016/j.jep.2021.113978. Epub 2021 Mar 11. J Ethnopharmacol. 2021. PMID: 33716082
-
Progranulin induces immune escape in breast cancer via up-regulating PD-L1 expression on tumor-associated macrophages (TAMs) and promoting CD8+ T cell exclusion.J Exp Clin Cancer Res. 2021 Jan 4;40(1):4. doi: 10.1186/s13046-020-01786-6. J Exp Clin Cancer Res. 2021. PMID: 33390170 Free PMC article.
-
CAA-derived IL-6 induced M2 macrophage polarization by activating STAT3.BMC Cancer. 2023 May 1;23(1):392. doi: 10.1186/s12885-023-10826-1. BMC Cancer. 2023. PMID: 37127625 Free PMC article.
-
Mechanisms of tumor-associated macrophages in breast cancer and treatment strategy.Front Immunol. 2025 Feb 28;16:1560393. doi: 10.3389/fimmu.2025.1560393. eCollection 2025. Front Immunol. 2025. PMID: 40092996 Free PMC article. Review.
-
Tumor-associated macrophages: An important player in breast cancer progression.Thorac Cancer. 2022 Feb;13(3):269-276. doi: 10.1111/1759-7714.14268. Epub 2021 Dec 15. Thorac Cancer. 2022. PMID: 34914196 Free PMC article. Review.
Cited by
-
FAT4 expression in peripheral blood mononuclear cells is associated with prognosis and immune cell infiltration in hepatocellular carcinoma.Sci Rep. 2023 Sep 21;13(1):15735. doi: 10.1038/s41598-023-42560-w. Sci Rep. 2023. PMID: 37735184 Free PMC article.
-
Network pharmacology to explore the novel anti-inflammatory mechanism of naringenin in intestinal ischemia/reperfusion injury.Front Immunol. 2025 Aug 8;16:1623080. doi: 10.3389/fimmu.2025.1623080. eCollection 2025. Front Immunol. 2025. PMID: 40861465 Free PMC article.
-
Matrix stiffness affects tumor-associated macrophage functional polarization and its potential in tumor therapy.J Transl Med. 2024 Jan 21;22(1):85. doi: 10.1186/s12967-023-04810-3. J Transl Med. 2024. PMID: 38246995 Free PMC article. Review.
-
Orchestration of Tumor-Associated Macrophages in the Tumor Cell-Macrophage-CD8+ T Cell Loop for Cancer Immunotherapy.Int J Biol Sci. 2025 Jun 12;21(9):4098-4116. doi: 10.7150/ijbs.115932. eCollection 2025. Int J Biol Sci. 2025. PMID: 40607254 Free PMC article. Review.
-
Combined anti-PD-L1 and anti-VEGFR2 therapy promotes the antitumor immune response in GBM by reprogramming tumor microenvironment.Cell Death Discov. 2025 Apr 3;11(1):136. doi: 10.1038/s41420-025-02427-7. Cell Death Discov. 2025. PMID: 40180890 Free PMC article.
References
-
- Brook N, Brook E, Dharmarajan A, Dass CR, Chan A. Breast cancer bone metastases: pathogenesis and therapeutic targets. Int J Biochem Cell Biol. 2018;96:63‐78. - PubMed
-
- Jafari SH, Saadatpour Z, Salmaninejad A, et al. Breast cancer diagnosis: imaging techniques and biochemical markers. J Cell Physiol. 2018;233(7):5200‐5213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

