Adipose Tissue Dysfunction in Polycystic Ovary Syndrome
- PMID: 37329216
- PMCID: PMC10735305
- DOI: 10.1210/clinem/dgad356
Adipose Tissue Dysfunction in Polycystic Ovary Syndrome
Abstract
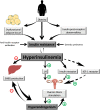
Purpose: Polycystic ovary syndrome (PCOS) is a complex genetic trait and the most common endocrine disorder of women, clinically evident in 5% to 15% of reproductive-aged women globally, with associated cardiometabolic dysfunction. Adipose tissue (AT) dysfunction appears to play an important role in the pathophysiology of PCOS even in patients who do not have excess adiposity.
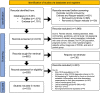
Methods: We undertook a systematic review concerning AT dysfunction in PCOS, and prioritized studies that assessed AT function directly. We also explored therapies that targeted AT dysfunction for the treatment of PCOS.
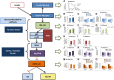
Results: Various mechanisms of AT dysfunction in PCOS were identified including dysregulation in storage capacity, hypoxia, and hyperplasia; impaired adipogenesis; impaired insulin signaling and glucose transport; dysregulated lipolysis and nonesterified free fatty acids (NEFAs) kinetics; adipokine and cytokine dysregulation and subacute inflammation; epigenetic dysregulation; and mitochondrial dysfunction and endoplasmic reticulum and oxidative stress. Decreased glucose transporter-4 expression and content in adipocytes, leading to decreased insulin-mediated glucose transport in AT, was a consistent abnormality despite no alterations in insulin binding or in IRS/PI3K/Akt signaling. Adiponectin secretion in response to cytokines/chemokines is affected in PCOS compared to controls. Interestingly, epigenetic modulation via DNA methylation and microRNA regulation appears to be important mechanisms underlying AT dysfunction in PCOS.
Conclusion: AT dysfunction, more than AT distribution and excess adiposity, contributes to the metabolic and inflammation abnormalities of PCOS. Nonetheless, many studies provided contradictory, unclear, or limited data, highlighting the urgent need for additional research in this important field.
Keywords: DNA methylation; GLP-1R agonists; GLUT-4; PCOS; adipokines; adiponectin; adipose tissue; cytokines; diet; exercise; fat; hyperandrogenism; inflammation; insulin signaling; metabolic dysfunction; metabolic syndrome; metformin; miRNAs; thiazolidinediones; weight loss.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841‐2855. - PubMed
-
- Riestenberg C, Jagasia A, Markovic D, Buyalos RP, Azziz R. Health care-related economic burden of polycystic ovary syndrome in the United States: pregnancy-related and long-term health consequences. J Clin Endocrinol Metab. 2022;107(2):575‐585. - PubMed
-
- Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6‐15. - PubMed
-
- Azziz R, Carmina E, Chen ZJ, et al. . Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2(1):16057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

