Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study
- PMID: 37329891
- PMCID: PMC11298287
- DOI: 10.1016/S1470-2045(23)00215-2
Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study
Abstract
Background: Approximately 15-20% of advanced gastric and gastro-oesophageal junction cancers overexpress HER2. In DESTINY-Gastric01, the HER2-targeted antibody-drug conjugate trastuzumab deruxtecan improved response and overall survival versus chemotherapy in patients from Japan and South Korea with locally advanced or metastatic HER2-positive gastric or gastro-oesophageal junction cancer whose disease progressed after two lines of previous therapy including trastuzumab. Here, we report primary and updated analyses of the single-arm, phase 2 DESTINY-Gastric02 trial, which aimed to examine trastuzumab deruxtecan in patients living in the USA and Europe.
Methods: DESTINY-Gastric02 is a single-arm, phase 2 study in adult patients from 24 study sites in the USA and Europe (Belgium, Spain, Italy, and the UK). Eligible patients were aged at least 18 years and had an Eastern Cooperative Oncology Group performance status of 0 or 1, pathologically documented unresectable or metastatic gastric or gastro-oesophageal junction cancer, progressive disease on or after first-line therapy with a trastuzumab-containing regimen, with at least one measurable lesion per Response Evaluation Criteria in Solid Tumours (version 1.1), and centrally confirmed HER2-positive disease on a postprogression biopsy. Patients were given 6·4 mg/kg of trastuzumab deruxtecan intravenously every 3 weeks until disease progression, withdrawal by patient, physician decision, or death. The primary endpoint was confirmed objective response rate by independent central review. The primary endpoint and safety were assessed in the full analysis set (ie, participants who received at least one dose of study drug). Here, we report the primary analysis of this study, with a data cutoff of April 9, 2021, and an updated analysis, with a data cutoff of Nov 8, 2021. This trial is registered with ClinicalTrials.gov, NCT04014075, and is ongoing.
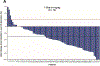
Findings: Between Nov 26, 2019, and Dec 2, 2020, 89 patients were screened and 79 were enrolled and subsequently treated with trastuzumab deruxtecan (median age 60·7 years [IQR 52·0-68·3], 57 [72%] of 79 were male, 22 [28%] were female, 69 [87%] were White, four [5%] were Asian, one [1%] was Black or African American, one [1%] was Native Hawaiian or Pacific Islander, one had missing race, and three [4%] were other races). At the primary analysis (median follow-up 5·9 months [IQR 4·6-8·6 months]), confirmed objective response was reported in 30 (38% [95% CI 27·3-49·6]) of 79 patients, including three (4%) complete responses and 27 (34%) partial responses, as assessed by independent central review. As of data cutoff for the updated analysis (median follow-up 10·2 months [IQR 5·6-12·9]), a confirmed objective response was reported in 33 (42% [95% CI 30·8-53·4]) of 79 patients, including four (5%) complete responses and 29 (37%) partial responses, as assessed by independent central review. The most common grade 3 or worse treatment-emergent adverse events were anaemia (11 [14%]), nausea (six [8%]), decreased neutrophil count (six [8%]), and decreased white blood cell count (five [6%]). Drug-related serious treatment-emergent adverse events occurred in ten patients (13%). Deaths determined to be associated with study treatment occurred in two patients (3%) and were due to interstitial lung disease or pneumonitis.
Interpretation: These clinically meaningful results support the use of trastuzumab deruxtecan as second-line therapy in patients with HER2-positive advanced gastric or gastro-oesophageal junction cancer.
Funding: Daiichi Sankyo and AstraZeneca.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests EVC has received research grants paid to his institution from Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Ipsen, Lilly, Merck Sharp & Dohme, Merck, Novartis, Roche, and Servier and has participated in advisory boards for AbbVie, Array, Astellas, AstraZeneca, Bayer, Beigene, Biocartis, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Halozyme, GlaxoSmithKline, Helsinn, Incyte, Ipsen, Janssen Research, Lilly, Merck Sharp & Dohme, Merck, Mirati, Novartis, Pierre Fabre, Roche, Seagen, Servier, Sirtex, Terumo, Taiho, TRIGR, and Zymeworks. ES has received consulting fees from Amal Therapeutics, AstraZeneca, Bristol Myers Squibb, Beigene, Daiichi Sankyo, Merck, Novartis, Pfizer, Roche, Servier, Turning Point Therapeutics, and Zymeworks; speaker payments or honoraria from Amgen, Bristol Myers Squibb, Novartis, and Servier; support for meeting attendance or travel from Amgen, Bristol Myers Squibb, and Servier; and participated on a data safety monitoring board or advisory board for Amgen, AstraZeneca, and Beigene; and held a leadership role (co-chair) of the EORTC GI Trials Group Gastric Cancer Task Force. IC has received research funding from Eli Lilly and Janssen-Cilag, has participated in advisory boards for Eli Lilly, Bristol Myers Squibb, Merck Sharp & Dohme, Roche, Merck Serono, AstraZeneca, OncXerna, Pierre Fabre, Boehringer Ingelheim, Incyte, Astellas, GlaxoSmithKline, Sotio, Eisai, Daiichi Sankyo, Taiho, Servier, Seagen, and Turning Point Therapeutics; speaker payments or honoraria from Eli Lilly, Eisai, and Servier; and has received support for meeting attendance or travel from Bristol Myers Squibb. HP has received grants or contracts from Adlai Nortye USA, Alpine Immune Sciences, Ambrx, Amgen, Aprea Therapeutics, Array BioPharma, Bayer, BeiGene, BJ Bioscience, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Elicio Therapeutics, EMD Serono, Exelixis, Genentech, Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Hoffman-LaRoche, Hutchison MediPharma, ImmuneOncia Therapeutics, Incyte, Jounce Therapeutics, Mabspace Biosciences, MacroGenics, Medimmune, Medivation, Merck, Millennium, Mirati Therapeutics, Novartis Pharmaceuticals, Oncologie, Pfizer, PsiOxus Therapeutics, Puma Biotechnology, Regeneron Pharmaceuticals, RePare Therapeutics, Seagen, Synermore Biologics, Taiho Pharmaceutical, TopAlliance Biosciences, Turning Point Therapeutics, Vedanta Biosciences, Xencor, and participated on a data safety monitoring board or advisory board for Jacobio. SS has participated in advisory boards for Agenus, AstraZeneca, Bayer, Bristol Myers Squibb, CheckmAb, Daiichi Sankyo, Guardant Health, Menarini, Merck, Novartis, Pierre Fabre, Roche-Genentech, and Seagen. SL has received research funding paid to her institution from Daiichi Sankyo, Amgen, Merck Serono, Bayer, Roche, Lilly, AstraZeneca, and Bristol Myers Squibb; consulting fees from Amgen, Merck Serono, Lilly, AstraZeneca, Incyte, Daiichi Sankyo, Bristol Myers Squibb, Servier, and Merck Sharp & Dohme; and speaker payments or honoraria from Roche, Lilly, Bristol Myers Squibb, Servier, Merck Serono, Pierre Fabre, GlaxoSmithKline, and Amgen. ZAW has received consulting fees from Merck, Ibsen, Lilly, Five Prime, QED, Molecular Templates, Daiichi Sankyo, AstraZeneca, Bayer, and Novartis; support for meeting attendance or travel from Lilly, Merck, Novartis, and Daiichi Sankyo; and participated on a data safety monitoring board or advisory board for Array. JC has received research funding paid to his institution from Daiichi Sankyo, Merck, and Brooklyn Immunotherapeutics; consulting fees from Lilly, Merck, AstraZeneca, Foundation Medicine, Daiichi Sankyo, Macrogenics, Amgen, Ono Pharmaceutical, Bristol Myers Squibb, Astellas, Turning Point Therapeutics, Silverback Therapeutics, Novartis, Coherus Biosciences, Geneos, and Roche; speaker payments or honoraria from Merck and Bristol Myers Squibb; and participated on a data safety monitoring board for Yiviva. YJ has received grants or contracts from Bayer, Bristol Myers Squibb, Cycle for Survival, Department of Defense, Eli Lilly, Fred's Team, Genentech Roche, Merck, NCI, and RGENIX; speaker payments or honoraria from Amerisource Bergen, Arcus Biosciences, AstraZeneca, Basilea Pharmaceutica, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Geneos Therapeutics, GlaxoSmithKline, Imedex, Imugene, Lynx Health, Merck, Merck Serono, Michael J Hennessy Associates, Paradigm Medical Communications, PeerView Institute, Pfizer, Research to Practice, RGENIX, Seagen, Silverback Therapeutics, and Zymeworks; and stock options from RGENIX. FB was an employee at the time of this study and held stocks or stock options in Daiichi Sankyo. YK, AQ, and JS are employees of Daiichi Sankyo. GK has received research funding paid to his institution from Arog, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Merck, Oncolys, Pieris, and Zymeworks; consulting fees from Apexigen, AstraZeneca, Bristol Myers Squibb, Merck, Pieris, and Zymeworks; support for meeting attendance or travel from Bristol Myers Squibb, Merck, and Pieris; and participated on a data safety monitoring board or advisory board for Zymeworks. MdB and JA declare no competing interests.
Figures



References
-
- Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 2020; 382: 2419–30. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–97. - PubMed
-
- Janjigian YY, Kawazoe A, Yanez PE, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: Initial findings of the global phase 3 KEYNOTE-811 study. J Clin Oncol 2021; 39(15 suppl). Abstract 4013.
-
- US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for HER2-positive gastric cancer. Published May 5, 2021. Accessed March 8, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grant...
-
- Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017; 18: 640–53. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

