Role of HIF1α and HIF2α in Cre Recombinase-Induced Retinal Pigment Epithelium Pathology and Its Secondary Effect on Choroidal Neovascularization
- PMID: 37330004
- PMCID: PMC12178331
- DOI: 10.1016/j.ajpath.2023.05.017
Role of HIF1α and HIF2α in Cre Recombinase-Induced Retinal Pigment Epithelium Pathology and Its Secondary Effect on Choroidal Neovascularization
Abstract
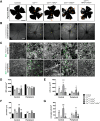
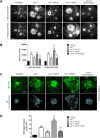
CreTrp1 mice are widely used for conditional retinal pigment epithelium (RPE) gene function studies. Like other Cre/LoxP models, phenotypes in CreTrp1 mice can be affected by Cre-mediated cellular toxicity, leading to RPE dysfunction, altered morphology and atrophy, activation of innate immunity, and consequent impairment of photoreceptor function. These effects are common among the age-related alterations of RPE that feature in early/intermediate forms of age-related macular degeneration. This article characterizes Cre-mediated pathology in the CreTrp1 line to elucidate the impact of RPE degeneration on both developmental and pathologic choroidal neovascularization. Nonredundant roles of the two major components of the hypoxia-inducible factor (HIF) family of transcription regulators, HIF1α and HIF2α, were identified. Genetic ablation of Hif1a protected against Cre-induced degeneration of RPE and choroid, whereas ablation of Hif2a exacerbated this degeneration. Furthermore, HIF1α deficiency protected CreTrp1 mice against laser-induced choroidal neovascularization, whereas HIF2α deficiency exacerbated the phenotype. Cre-mediated degeneration of the RPE in CreTrp1 mice offers an opportunity to investigate the impact of hypoxia signaling in the context of RPE degeneration. These findings indicate that HIF1α promotes Cre recombinase-mediated RPE degeneration and laser-induced choroidal neovascularization, whereas HIF2α is protective.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Stromal-Like Cells and Retinal Pigment Epithelium Modulate Choroidal Sprouting Through Galectin-1-Dependent and Independent Pathways.FASEB J. 2025 May 31;39(10):e70671. doi: 10.1096/fj.202403181R. FASEB J. 2025. PMID: 40407849
-
AIM2 activation mediated by RIPK1/3-dependent mitochondrial DNA release drives Aβ1-40-Induced retinal pigment epithelium injury.Cell Commun Signal. 2025 Jun 21;23(1):301. doi: 10.1186/s12964-025-02294-w. Cell Commun Signal. 2025. PMID: 40544279 Free PMC article.
-
Feedback regulation between histone lactylation and ALKBH3-mediated glycolysis regulates age-related macular degeneration pathology.Proc Natl Acad Sci U S A. 2025 Jun 17;122(24):e2416046122. doi: 10.1073/pnas.2416046122. Epub 2025 Jun 10. Proc Natl Acad Sci U S A. 2025. PMID: 40493193
-
Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update.Ophthalmology. 2003 Jan;110(1):15-21; quiz 22-3. doi: 10.1016/s0161-6420(02)01563-4. Ophthalmology. 2003. PMID: 12511340
-
Photodynamic therapy for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2003;(2):CD002030. doi: 10.1002/14651858.CD002030. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2005 Oct 19;(4):CD002030. doi: 10.1002/14651858.CD002030.pub2. PMID: 12804420 Updated.
Cited by
-
Ablating VHL in rod photoreceptors modulates RPE glycolysis and improves preclinical model of retinitis pigmentosa.J Clin Invest. 2025 Feb 12;135(7):e185796. doi: 10.1172/JCI185796. J Clin Invest. 2025. PMID: 39932789 Free PMC article.
-
Deubiquitinating enzyme mutagenesis screens identify a USP43-dependent HIF-1 transcriptional response.EMBO J. 2024 Sep;43(17):3677-3709. doi: 10.1038/s44318-024-00166-6. Epub 2024 Jul 15. EMBO J. 2024. PMID: 39009674 Free PMC article.
References
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. - PubMed
-
- Zhao S., Overbeek P. Regulation of choroid development by the retinal pigment epithelium. Mol Vis. 2001;7:277–282. - PubMed
-
- Marmorstein A.D., Finnemann S.C., Bonilha V.L., Rodriguez-Boulan E. Morphogenesis of the retinal pigment epithelium: toward understanding retinal degenerative diseases. Ann N Y Acad Sci. 1998;857:1–12. - PubMed
LinkOut - more resources
Full Text Sources

