The NAD salvage pathway in mesenchymal cells is indispensable for skeletal development in mice
- PMID: 37330524
- PMCID: PMC10276814
- DOI: 10.1038/s41467-023-39392-7
The NAD salvage pathway in mesenchymal cells is indispensable for skeletal development in mice
Abstract
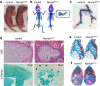
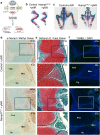
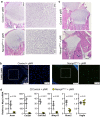
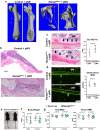
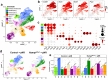
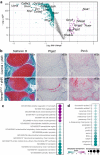
NAD is an essential co-factor for cellular energy metabolism and multiple other processes. Systemic NAD+ deficiency has been implicated in skeletal deformities during development in both humans and mice. NAD levels are maintained by multiple synthetic pathways but which ones are important in bone forming cells is unknown. Here, we generate mice with deletion of Nicotinamide Phosphoribosyltransferase (Nampt), a critical enzyme in the NAD salvage pathway, in all mesenchymal lineage cells of the limbs. At birth, NamptΔPrx1 exhibit dramatic limb shortening due to death of growth plate chondrocytes. Administration of the NAD precursor nicotinamide riboside during pregnancy prevents the majority of in utero defects. Depletion of NAD post-birth also promotes chondrocyte death, preventing further endochondral ossification and joint development. In contrast, osteoblast formation still occurs in knockout mice, in line with distinctly different microenvironments and reliance on redox reactions between chondrocytes and osteoblasts. These findings define a critical role for cell-autonomous NAD homeostasis during endochondral bone formation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

