Screening macroalgae for mitigation of enteric methane in vitro
- PMID: 37330586
- PMCID: PMC10276865
- DOI: 10.1038/s41598-023-36359-y
Screening macroalgae for mitigation of enteric methane in vitro
Abstract
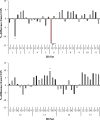
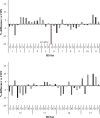
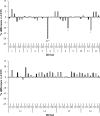
This study investigated the effects of 67 species of macroalgae on methanogenesis and rumen fermentation in vitro. Specimens were analyzed for their effect on ruminal fermentation and microbial community profiles. Incubations were carried out in an automated gas production system for 24-h and macroalgae were tested at 2% (feed dry matter basis) inclusion rate. Methane yield was decreased 99% by Asparagopsis taxiformis (AT) when compared with the control. Colpomenia peregrina also decreased methane yield 14% compared with control; no other species influenced methane yield. Total gas production was decreased 14 and 10% by AT and Sargassum horneri compared with control, respectively. Total volatile fatty acid (VFA) concentration was decreased between 5 and 8% by 3 macroalgae, whereas AT reduced it by 10%. Molar proportion of acetate was decreased 9% by AT, along with an increase in propionate by 14%. Asparagopsis taxiformis also increased butyrate and valerate molar proportions by 7 and 24%, respectively, whereas 3 macroalgae species decreased molar proportion of butyrate 3 to 5%. Vertebrata lanosa increased ammonia concentration, whereas 3 other species decreased it. Inclusion of AT decreased relative abundance of Prevotella, Bacteroidales, Firmicutes and Methanobacteriaceae, whereas Clostridium, Anaerovibrio and Methanobrevibacter were increased. Specific gene activities for Methanosphaera stadtmane and Methanobrevibacter ruminantium were decreased by AT inclusion. In this in vitro study, Asparagopsis taxiformis was most effective in decreasing methane concentration and yield, but also decreased total gas production and VFA concentration which indicates overall inhibition of ruminal fermentation. No other macroalgae were identified as potential mitigants of enteric methane.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- EPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks. Accessed April 15, 2022. https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-a.... (2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

