Examining protective effects of SARS-CoV-2 neutralizing antibodies after vaccination or monoclonal antibody administration
- PMID: 37330602
- PMCID: PMC10276829
- DOI: 10.1038/s41467-023-39292-w
Examining protective effects of SARS-CoV-2 neutralizing antibodies after vaccination or monoclonal antibody administration
Abstract
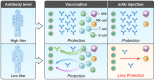
While new vaccines for SARS-CoV-2 are authorized based on neutralizing antibody (nAb) titer against emerging variants of concern, an analogous pathway does not exist for preventative monoclonal antibodies. In this work, nAb titers were assessed as correlates of protection against COVID-19 in the casirivimab + imdevimab monoclonal antibody (mAb) prevention trial (ClinicalTrials.gov #NCT4452318) and in the mRNA-1273 vaccine trial (ClinicalTrials.gov #NCT04470427). In the mAb trial, protective efficacy of 92% (95% confidence interval (CI): 84%, 98%) is associated with a nAb titer of 1000 IU50/ml, with lower efficacy at lower nAb titers. In the vaccine trial, protective efficacies of 93% [95% CI: 91%, 95%] and 97% (95% CI: 95%, 98%) are associated with nAb titers of 100 and 1000 IU50/ml, respectively. These data quantitate a nAb titer correlate of protection for mAbs benchmarked alongside vaccine induced nAb titers and support nAb titer as a surrogate endpoint for authorizing new mAbs.
Trial registration: ClinicalTrials.gov NCT04452318 NCT04470427.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
D.F., J.F., M.P.F., D.M., A.M., H.M.E.S., H.J., N.D.-R., D.B., Y.F., and M.M. have no competing interests to declare. M.P.O. is an employee, has stock options, a patent pending, and license and royalties with Regeneron Pharmaceuticals, Inc. G.A.H. is an employee and shareholder of Regeneron and is listed on pending patents for the REGEN-COV antibody cocktail. A.H. owns stock in Regeneron and Pfizer. K.-C.C. and E.F.-N. are employees and shareholders of Regeneron. K.C.T. and F.I. are employees and shareholders of Regeneron and are listed on a pending patent. L.R.B. is the Deputy Editor for the New England Journal of Medicine and has grants from the Bill and Melinda Gates Foundation, Harvard Medical School, the National Institutes of Health, and the Wellcome Trust. J.M. is an employee of and has stock options and stock grants from Moderna. H.Z. is an employee of and has stock options from Moderna. W.D. is an employee of Moderna. P.G.B. will be serving as an unpaid advisor on Moderna’s Zika Vaccine Advisory Board. M.S.C. serves on the scientific advisory boards of Aerium, ModexX, and Atea and has consulting roles with Astra Zenica and GSK.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

