Human amygdala involvement in Alzheimer's disease revealed by stereological and dia-PASEF analysis
- PMID: 37331354
- PMCID: PMC10467039
- DOI: 10.1111/bpa.13180
Human amygdala involvement in Alzheimer's disease revealed by stereological and dia-PASEF analysis
Abstract
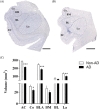
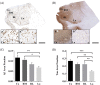
Alzheimer's disease (AD) is characterized by the accumulation of pathological amyloid-β (Aβ) and Tau proteins. According to the prion-like hypothesis, both proteins can seed and disseminate through brain regions through neural connections and glial cells. The amygdaloid complex (AC) is involved early in the disease, and its widespread connections with other brain regions indicate that it is a hub for propagating pathology. To characterize changes in the AC as well as the involvement of neuronal and glial cells in AD, a combined stereological and proteomic analysis was performed in non-Alzheimer's disease and AD human samples. The synaptic alterations identified by proteomic data analysis could be related to the volume reduction observed in AD by the Cavalieri probe without neuronal loss. The pathological markers appeared in a gradient pattern with the medial region (cortical nucleus, Co) being more affected than lateral regions, suggesting the relevance of connections in the distribution of the pathology among different brain regions. Generalized astrogliosis was observed in every AC nucleus, likely related to deposits of pathological proteins. Astrocytes might mediate phagocytic microglial activation, whereas microglia might play a dual role since protective and toxic phenotypes have been described. These results highlight the potential participation of the amygdala in the disease spreading from/to olfactory areas, the temporal lobe and beyond. Proteomic data are available via ProteomeXchange with identifier PXD038322.
Keywords: BM88 antigen (BM88); antioxidant protein 2 (AOP2); calpactin II; calpactin-1 heavy chain (CAL1H); centaurin-alpha-1 (CENTA1); endonexin II (ENX2); nuclear chloride ion channel 27 (NCC27).
© 2023 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

