Reactogenicity, immunogenicity and breakthrough infections following heterologous or fractional second dose COVID-19 vaccination in adolescents (Com-COV3): A randomised controlled trial
- PMID: 37331429
- PMCID: PMC10275659
- DOI: 10.1016/j.jinf.2023.06.007
Reactogenicity, immunogenicity and breakthrough infections following heterologous or fractional second dose COVID-19 vaccination in adolescents (Com-COV3): A randomised controlled trial
Abstract
Background: This was the first study to investigate the reactogenicity and immunogenicity of heterologous or fractional second dose COVID-19 vaccine regimens in adolescents.
Methods: A phase II, single-blind, multi-centre, randomised-controlled trial recruited across seven UK sites from September to November 2021, with follow-up visits to August 2022. Healthy 12-to-16 years olds were randomised (1:1:1) to either 30 µg BNT162b2 (BNT-30), 10 µg BNT162b2 (BNT-10), or NVX-CoV2373 (NVX), 8 weeks after a first 30 µg dose of BNT162b2. The primary outcome was solicited systemic reactions in the week following vaccination. Secondary outcomes included immunogenicity and safety. 'Breakthrough infection' analyses were exploratory.
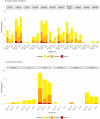
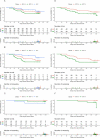
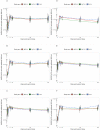
Findings: 148 participants were recruited (median age 14 years old, 62% female, 26% anti-nucleocapsid IgG seropositive pre-second dose); 132 participants received a second dose. Reactions were mostly mild-to-moderate, with lower rates in BNT-10 recipients. No vaccine-related serious adverse events occurred. Compared to BNT-30, at 28 days post-second dose anti-spike antibody responses were similar for NVX (adjusted geometric mean ratio [aGMR]) 1.09 95% confidence interval (CI): 0.84, 1.42] and lower for BNT-10 (aGMR 0.78 [95% CI: 0.61, 0.99]). For Omicron BA.1 and BA.2, the neutralising antibody titres for BNT-30 at day 28 were similar for BNT-10 (aGMR 1.0 [95% CI: 0.65, 1.54] and 1.02 [95% CI: 0.71, 1.48], respectively), but higher for NVX (aGMR 1.7 [95% CI: 1.07, 2.69] and 1.43 [95% CI: 0.96, 2.12], respectively). Compared to BNT-30, cellular immune responses were greatest for NVX (aGMR 1.73 [95% CI: 0.94, 3.18]), and lowest for BNT-10 (aGMR 0.65 [95% CI: 0.37, 1.15]) at 14 days post-second dose. Cellular responses were similar across the study arms by day 236 post-second dose. Amongst SARS-CoV-2 infection naïve participants, NVX participants had an 89% reduction in risk of self-reported 'breakthrough infection' compared to BNT-30 (adjusted hazard ratio [aHR] 0.11 [95% CI: 0.01, 0.86]) up until day 132 after second dose. BNT-10 recipients were more likely to have a 'breakthrough infection' compared to BNT-30 (aHR 2.14 [95% CI: 1.02, 4.51]) up to day 132 and day 236 post-second dose. Antibody responses at 132 and 236 days after second dose were similar for all vaccine schedules.
Interpretation: Heterologous and fractional dose COVID-19 vaccine schedules in adolescents are safe, well-tolerated and immunogenic. The enhanced performance of the heterologous schedule using NVX-CoV2373 against the Omicron SARS-CoV-2 variant suggests this mRNA prime and protein-subunit boost schedule may provide a greater breadth of protection than the licensed homologous schedule.
Funding: National Institute for Health Research and Vaccine Task Force.
Trial registration: International Standard Randomised Controlled Trial Number registry: 12348322.
Keywords: Adolescents; BNT162b2; Breakthrough infection; COVID-19; Heterologous; Immunisation; Immunity; NVXCoV2373; SARS-CoV-2; Vaccination.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: MDS acted until September 2022 on behalf of the University of Oxford as an Investigator on research studies funded or supported by the vaccine manufacturers GlaxoSmithKline, Janssen, AstraZeneca, Novavax, MCM vaccines and Pfizer. He received no direct personal benefit for this work. From September 2022 he has been an employee at Moderna Biotech and holds stock options in this company. SNF acts on behalf of University Hospital Southampton NHS Foundation Trust as an Investigator and/or providing consultative advice on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers including Janssen, Pfizer, AstraZeneca, GlaxoSmithKline, Novavax, Seqirus, Sanofi, Medimmune, Merck and Valneva vaccines and antimicrobials. He receives no personal financial payment for this work. KC acts on behalf of University Hospital Southampton NHS Foundation Trust as an investigator and/or providing consultative advice on studies funded or sponsored by vaccine manufacturers including AstraZeneca, GlaxoSmithKline, Janssen, Medimmune, Merck, Pfizer, Sanofi and Valneva. She receives no personal financial payment for this work. AMM acts on behalf of the University of Oxford as an investigator on research studies funded + /- sponsored by vaccine manufacturers including Pfizer, GlaxoSmithKline, Janssen, Valneva SE and Novavax. She receives no personal financial benefit for this work. PTH acts on behalf of St George’s University of London as an Investigator on clinical trials and studies of COVID-19 vaccines funded or sponsored by vaccine manufacturers including Janssen, Pfizer, AstraZeneca, Moderna, Novavax and Valneva. He receives no personal financial payment for this work. He is a member of the JCVI. JSN-V-T was seconded to the Department of Health and Social Care (DHSC) from October 2017-March 2022 as Deputy Chief Medical Officer, England, receiving no benefits, other than salary, for this work. Since leaving DHSC he has received a lecture fee from AstraZeneca and will undertake paid consulting for Moderna BioTech from 3rd May 2023. The views expressed in this paper are those of its authors and not necessarily those of DHSC or JCVI.
Figures


References
-
- Torjesen I. Covid-19: Omicron variant is linked to steep rise in hospital admissions of very young children. BMJ. 2022;376:o110. - PubMed
-
- Stuart A.S.V., Shaw R.H., Liu X., et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. The Lancet. 2022;399(10319):36–49. - PMC - PubMed
-
- World Health Organization. Interim recommendations for heterologous COVID-19 vaccine schedules,. 2021. 〈https://apps.who.int/iris/rest/bitstreams/1401003/retrieve〉 (accessed 17 June 2022).
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

