Why platelet mechanotransduction matters for hemostasis and thrombosis
- PMID: 37331517
- PMCID: PMC10529432
- DOI: 10.1016/j.jtha.2023.06.010
Why platelet mechanotransduction matters for hemostasis and thrombosis
Abstract
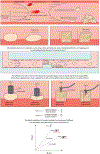
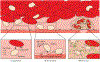
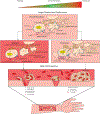
Mechanotransduction is the ability of cells to "feel" or sense their mechanical microenvironment and integrate and convert these physical stimuli into adaptive biochemical cellular responses. This phenomenon is vital for the physiology of numerous nucleated cell types to affect their various cellular processes. As the main drivers of hemostasis and clot retraction, platelets also possess this ability to sense the dynamic mechanical microenvironments of circulation and convert those signals into biological responses integral to clot formation. Like other cell types, platelets leverage their "hands" or receptors/integrins to mechanotransduce important signals in responding to vascular injury to achieve hemostasis. The clinical relevance of cellular mechanics and mechanotransduction is imperative as pathologic alterations or aberrant mechanotransduction in platelets has been shown to lead to bleeding and thrombosis. As such, the aim of this review is to provide an overview of the most recent research related to platelet mechanotransduction, from platelet generation to platelet activation, within the hemodynamic environment and clot contraction at the site of vascular injury, thereby covering the entire "life cycle" of platelets. Additionally, we describe the key mechanoreceptors in platelets and discuss the new biophysical techniques that have enabled the field to understand how platelets sense and respond to their mechanical microenvironment via those receptors. Finally, the clinical significance and importance of continued exploration of platelet mechanotransduction have been discussed as the key to better understanding of both thrombotic and bleeding disorders lies in a more complete mechanistic understanding of platelet function by way of mechanotransduction.
Keywords: biomechanics; blood platelets; hemostasis; mechanotransduction; thrombosis.
Copyright © 2023 International Society on Thrombosis and Haemostasis. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interests There are no competing interests to disclose.
Figures


Similar articles
-
Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation.Proc Natl Acad Sci U S A. 2014 Oct 7;111(40):14430-5. doi: 10.1073/pnas.1322917111. Epub 2014 Sep 22. Proc Natl Acad Sci U S A. 2014. PMID: 25246564 Free PMC article.
-
The platelet and the biophysical microenvironment: lessons from cellular mechanics.Thromb Res. 2014 Apr;133(4):532-7. doi: 10.1016/j.thromres.2013.12.037. Epub 2014 Jan 4. Thromb Res. 2014. PMID: 24440140 Free PMC article. Review.
-
Single-platelet nanomechanics measured by high-throughput cytometry.Nat Mater. 2017 Feb;16(2):230-235. doi: 10.1038/nmat4772. Epub 2016 Oct 10. Nat Mater. 2017. PMID: 27723740 Free PMC article.
-
Platelet Mechanotransduction.Annu Rev Biomed Eng. 2018 Jun 4;20:253-275. doi: 10.1146/annurev-bioeng-062117-121215. Annu Rev Biomed Eng. 2018. PMID: 29865873 Review.
-
Platelet packing density is an independent regulator of the hemostatic response to injury.J Thromb Haemost. 2018 May;16(5):973-983. doi: 10.1111/jth.13986. Epub 2018 Apr 2. J Thromb Haemost. 2018. PMID: 29488682 Free PMC article.
Cited by
-
Platelets as drivers of immunothrombosis in rheumatic diseases.Nat Rev Rheumatol. 2025 Aug;21(8):478-493. doi: 10.1038/s41584-025-01276-z. Epub 2025 Jul 7. Nat Rev Rheumatol. 2025. PMID: 40624395 Review.
-
Autoantibodies immuno-mechanically modulate platelet contractile force and bleeding risk.Nat Commun. 2024 Nov 25;15(1):10201. doi: 10.1038/s41467-024-54309-8. Nat Commun. 2024. PMID: 39587073 Free PMC article.
-
Biomedical engineering and interdisciplinary research in shaping tomorrow's medicine.iScience. 2025 Jul 2;28(7):112884. doi: 10.1016/j.isci.2025.112884. eCollection 2025 Jul 18. iScience. 2025. PMID: 40687809 Free PMC article.
-
Clot Retraction and Its Correlation with the Function of Platelet Integrin αIIbβ3.Biomedicines. 2023 Aug 23;11(9):2345. doi: 10.3390/biomedicines11092345. Biomedicines. 2023. PMID: 37760786 Free PMC article.
-
Biomechanical platelet activation: diseases that require a new class of antiplatelet therapeutics.Am J Physiol Cell Physiol. 2025 Jun 1;328(6):C1831-C1836. doi: 10.1152/ajpcell.00228.2025. Epub 2025 Apr 24. Am J Physiol Cell Physiol. 2025. PMID: 40272865 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

