Effects of sevoflurane on lung epithelial permeability in experimental models of acute respiratory distress syndrome
- PMID: 37331963
- PMCID: PMC10278282
- DOI: 10.1186/s12967-023-04253-w
Effects of sevoflurane on lung epithelial permeability in experimental models of acute respiratory distress syndrome
Abstract
Background: Preclinical studies in acute respiratory distress syndrome (ARDS) have suggested that inhaled sevoflurane may have lung-protective effects and clinical trials are ongoing to assess its impact on major clinical outcomes in patients with ARDS. However, the underlying mechanisms of these potential benefits are largely unknown. This investigation focused on the effects of sevoflurane on lung permeability changes after sterile injury and the possible associated mechanisms.
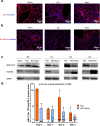
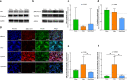
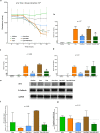
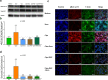
Methods: To investigate whether sevoflurane could decrease lung alveolar epithelial permeability through the Ras homolog family member A (RhoA)/phospho-Myosin Light Chain 2 (Ser19) (pMLC)/filamentous (F)-actin pathway and whether the receptor for advanced glycation end-products (RAGE) may mediate these effects. Lung permeability was assessed in RAGE-/- and littermate wild-type C57BL/6JRj mice on days 0, 1, 2, and 4 after acid injury, alone or followed by exposure at 1% sevoflurane. Cell permeability of mouse lung epithelial cells was assessed after treatment with cytomix (a mixture of TNFɑ, IL-1β, and IFNγ) and/or RAGE antagonist peptide (RAP), alone or followed by exposure at 1% sevoflurane. Levels of zonula occludens-1, E-cadherin, and pMLC were quantified, along with F-actin immunostaining, in both models. RhoA activity was assessed in vitro.
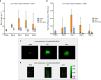
Results: In mice after acid injury, sevoflurane was associated with better arterial oxygenation, decreased alveolar inflammation and histological damage, and non-significantly attenuated the increase in lung permeability. Preserved protein expression of zonula occludens-1 and less increase of pMLC and actin cytoskeletal rearrangement were observed in injured mice treated with sevoflurane. In vitro, sevoflurane markedly decreased electrical resistance and cytokine release of MLE-12 cells, which was associated with higher protein expression of zonula occludens-1. Improved oxygenation levels and attenuated increase in lung permeability and inflammatory response were observed in RAGE-/- mice compared to wild-type mice, but RAGE deletion did not influence the effects of sevoflurane on permeability indices after injury. However, the beneficial effect of sevoflurane previously observed in wild-type mice on day 1 after injury in terms of higher PaO2/FiO2 and decreased alveolar levels of cytokines was not found in RAGE-/- mice. In vitro, RAP alleviated some of the beneficial effects of sevoflurane on electrical resistance and cytoskeletal rearrangement, which was associated with decreased cytomix-induced RhoA activity.
Conclusions: Sevoflurane decreased injury and restored epithelial barrier function in two in vivo and in vitro models of sterile lung injury, which was associated with increased expression of junction proteins and decreased actin cytoskeletal rearrangement. In vitro findings suggest that sevoflurane may decrease lung epithelial permeability through the RhoA/pMLC/F-actin pathway.
Keywords: Acute respiratory distress syndrome; Intracellular pathways; Junction proteins; Lung epithelial barrier function; Receptor for advanced glycation end-products; Sevoflurane.
© 2023. The Author(s).
Conflict of interest statement
No competing interests, other source of financial support, corporate involvement, patent holdings, etc. is to be declared for all authors.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

