Abcd1 deficiency accelerates cuprizone-induced oligodendrocyte loss and axonopathy in a demyelinating mouse model of X-linked adrenoleukodystrophy
- PMID: 37331971
- PMCID: PMC10276915
- DOI: 10.1186/s40478-023-01595-w
Abcd1 deficiency accelerates cuprizone-induced oligodendrocyte loss and axonopathy in a demyelinating mouse model of X-linked adrenoleukodystrophy
Abstract
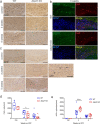
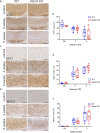
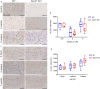
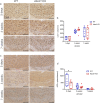
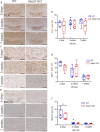
X-linked adrenoleukodystrophy (X-ALD), the most frequent, inherited peroxisomal disease, is caused by mutations in the ABCD1 gene encoding a peroxisomal lipid transporter importing very long-chain fatty acids (VLCFAs) from the cytosol into peroxisomes for degradation via β-oxidation. ABCD1 deficiency results in accumulation of VLCFAs in tissues and body fluids of X-ALD patients with a wide range of phenotypic manifestations. The most severe variant, cerebral X-ALD (CALD) is characterized by progressive inflammation, loss of the myelin-producing oligodendrocytes and demyelination of the cerebral white matter. Whether the oligodendrocyte loss and demyelination in CALD are caused by a primary cell autonomous defect or injury to oligodendrocytes or by a secondary effect of the inflammatory reaction remains unresolved. To address the role of X-ALD oligodendrocytes in demyelinating pathophysiology, we combined the Abcd1 deficient X-ALD mouse model, in which VLCFAs accumulate without spontaneous demyelination, with the cuprizone model of toxic demyelination. In mice, the copper chelator cuprizone induces reproducible demyelination in the corpus callosum, followed by remyelination upon cuprizone removal. By immunohistochemical analyses of oligodendrocytes, myelin, axonal damage and microglia activation during de-and remyelination, we found that the mature oligodendrocytes of Abcd1 KO mice are more susceptible to cuprizone-induced cell death compared to WT mice in the early demyelinating phase. Furthermore, this effect was mirrored by a greater extent of acute axonal damage during demyelination in the KO mice. Abcd1 deficiency did not affect the function of microglia in either phase of the treatment. Also, the proliferation and differentiation of oligodendrocyte precursor cells and remyelination progressed at similar rates in both genotypes. Taken together, our findings point to an effect of Abcd1 deficiency on mature oligodendrocytes and the oligodendrocyte-axon unit, leading to increased vulnerability in the context of a demyelinating insult.
Keywords: Abcd1 KO; Axonopathy; Cuprizone; Demyelination; Microglia; Oligodendrocytes; Peroxisome; X-ALD.
© 2023. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

