Astrocyte-mediated mechanisms contribute to traumatic brain injury pathology
- PMID: 37332001
- PMCID: PMC10526985
- DOI: 10.1002/wsbm.1622
Astrocyte-mediated mechanisms contribute to traumatic brain injury pathology
Abstract
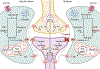
Astrocytes respond to traumatic brain injury (TBI) with changes to their molecular make-up and cell biology, which results in changes in astrocyte function. These changes can be adaptive, initiating repair processes in the brain, or detrimental, causing secondary damage including neuronal death or abnormal neuronal activity. The response of astrocytes to TBI is often-but not always-accompanied by the upregulation of intermediate filaments, including glial fibrillary acidic protein (GFAP) and vimentin. Because GFAP is often upregulated in the context of nervous system disturbance, reactive astrogliosis is sometimes treated as an "all-or-none" process. However, the extent of astrocytes' cellular, molecular, and physiological adjustments is not equal for each TBI type or even for each astrocyte within the same injured brain. Additionally, new research highlights that different neurological injuries and diseases result in entirely distinctive and sometimes divergent astrocyte changes. Thus, extrapolating findings on astrocyte biology from one pathological context to another is problematic. We summarize the current knowledge about astrocyte responses specific to TBI and point out open questions that the field should tackle to better understand how astrocytes shape TBI outcomes. We address the astrocyte response to focal versus diffuse TBI and heterogeneity of reactive astrocytes within the same brain, the role of intermediate filament upregulation, functional changes to astrocyte function including potassium and glutamate homeostasis, blood-brain barrier maintenance and repair, metabolism, and reactive oxygen species detoxification, sex differences, and factors influencing astrocyte proliferation after TBI. This article is categorized under: Neurological Diseases > Molecular and Cellular Physiology.
Keywords: TBI; astrocyte; gliosis; heterogeneity; traumatic brain injury.
© 2023 The Authors. WIREs Mechanisms of Disease published by Wiley Periodicals LLC.
Conflict of interest statement
Conflict of Interest
There is no conflict of interest.
Figures
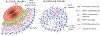
References
-
- Abou-Abbass H, Bahmad H, Ghandour H, Fares J, Wazzi-Mkahal R, Yacoub B, Darwish H, Mondello S, Harati H, el Sayed MJ, Tamim H, & Kobeissy F (2016). Epidemiology and clinical characteristics of traumatic brain injury in Lebanon: A systematic review. Medicine, 95(47), e5342. 10.1097/MD.0000000000005342 - DOI - PMC - PubMed
-
- Amorini AM, Lazzarino G, di Pietro V, Signoretti S, Lazzarino G, Belli A, & Tavazzi B (2016). Metabolic, enzymatic and gene involvement in cerebral glucose dysmetabolism after traumatic brain injury. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1862(4), 679–687. 10.1016/j.bbadis.2016.01.023 - DOI - PubMed
-
- Arneson D, Zhang G, Ahn IS, Ying Z, Diamante G, Cely I, Palafox-Sanchez V, Gomez-Pinilla F, & Yang X (2022). Systems spatiotemporal dynamics of traumatic brain injury at single-cell resolution reveals humanin as a therapeutic target. Cell. Mol. Life Sci, 79(9), 480. 10.1007/s00018-022-04495-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

