Visible and Near-Infrared Spectroscopy Enables Differentiation of Normal and Early Osteoarthritic Human Knee Joint Articular Cartilage
- PMID: 37332006
- PMCID: PMC10518273
- DOI: 10.1007/s10439-023-03261-7
Visible and Near-Infrared Spectroscopy Enables Differentiation of Normal and Early Osteoarthritic Human Knee Joint Articular Cartilage
Abstract
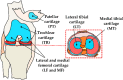
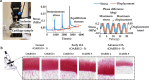
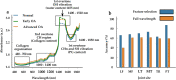
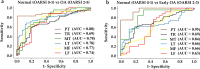
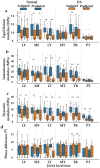
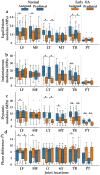
Osteoarthritis degenerates cartilage and impairs joint function. Early intervention opportunities are missed as current diagnostic methods are insensitive to early tissue degeneration. We investigated the capability of visible light-near-infrared spectroscopy (Vis-NIRS) to differentiate normal human cartilage from early osteoarthritic one. Vis-NIRS spectra, biomechanical properties and the state of osteoarthritis (OARSI grade) were quantified from osteochondral samples harvested from different anatomical sites of human cadaver knees. Two support vector machines (SVM) classifiers were developed based on the Vis-NIRS spectra and OARSI scores. The first classifier was designed to distinguish normal (OARSI: 0-1) from general osteoarthritic cartilage (OARSI: 2-5) to check the general suitability of the approach yielding an average accuracy of 75% (AUC = 0.77). Then, the second classifier was designed to distinguish normal from early osteoarthritic cartilage (OARSI: 2-3) yielding an average accuracy of 71% (AUC = 0.73). Important wavelength regions for differentiating normal from early osteoarthritic cartilage were related to collagen organization (wavelength region: 400-600 nm), collagen content (1000-1300 nm) and proteoglycan content (1600-1850 nm). The findings suggest that Vis-NIRS allows objective differentiation of normal and early osteoarthritic tissue, e.g., during arthroscopic repair surgeries.
Keywords: Biomechanics; Cartilage; Machine learning; Near-infrared spectroscopy; Osteoarthritis; Visible light spectroscopy.
© 2023. The Author(s).
Conflict of interest statement
The authors have no conflict of interest.
Figures


Similar articles
-
Near-infrared spectroscopy enables quantitative evaluation of human cartilage biomechanical properties during arthroscopy.Osteoarthritis Cartilage. 2019 Aug;27(8):1235-1243. doi: 10.1016/j.joca.2019.04.008. Epub 2019 Apr 23. Osteoarthritis Cartilage. 2019. PMID: 31026649
-
The evolving large-strain shear responses of progressively osteoarthritic human cartilage.Osteoarthritis Cartilage. 2019 May;27(5):810-822. doi: 10.1016/j.joca.2018.12.025. Epub 2019 Jan 18. Osteoarthritis Cartilage. 2019. PMID: 30660720
-
OARSI osteoarthritis cartilage histopathology assessment system: A biomechanical evaluation in the human knee.J Orthop Res. 2016 Jan;34(1):135-40. doi: 10.1002/jor.23010. Epub 2015 Aug 27. J Orthop Res. 2016. PMID: 26250350
-
Sonographic imaging of normal and osteoarthritic cartilage.Semin Arthritis Rheum. 1999 Jun;28(6):398-403. doi: 10.1016/s0049-0172(99)80005-5. Semin Arthritis Rheum. 1999. PMID: 10406407 Review.
-
Biochemical, biomechanical and histological properties of osteoarthritic porcine knee cartilage: implications for osteochondral transplantation.Arch Orthop Trauma Surg. 2008 Jan;128(1):61-70. doi: 10.1007/s00402-007-0360-5. Epub 2007 May 16. Arch Orthop Trauma Surg. 2008. PMID: 17505836 Review.
Cited by
-
Holistic vibrational spectromics assessment of human cartilage for osteoarthritis diagnosis.Biomed Opt Express. 2024 Jun 13;15(7):4264-4280. doi: 10.1364/BOE.520171. eCollection 2024 Jul 1. Biomed Opt Express. 2024. PMID: 39022535 Free PMC article.
-
Ultraviolet-Visible-Near Infrared Spectroscopy May Aid in the Qualitative Assessment of Early-Stage Cartilage Degradation.Arthrosc Sports Med Rehabil. 2024 Jan 9;6(1):100842. doi: 10.1016/j.asmr.2023.100842. eCollection 2024 Feb. Arthrosc Sports Med Rehabil. 2024. PMID: 38414840 Free PMC article.
-
Harnessing Raman spectroscopy and multimodal imaging of cartilage for osteoarthritis diagnosis.Sci Rep. 2024 Dec 28;14(1):31466. doi: 10.1038/s41598-024-83155-3. Sci Rep. 2024. PMID: 39733214 Free PMC article.
-
Orientation-Independent T2 Mapping Enhances MRI-Based Cartilage Characterization.Ann Biomed Eng. 2025 Jun 17. doi: 10.1007/s10439-025-03774-3. Online ahead of print. Ann Biomed Eng. 2025. PMID: 40528094
-
Knee Osteoarthritis Diagnosis: Future and Perspectives.Biomedicines. 2025 Jul 4;13(7):1644. doi: 10.3390/biomedicines13071644. Biomedicines. 2025. PMID: 40722715 Free PMC article. Review.
References
-
- Belousov AI, Verzakov SA, Von Frese J. A flexible classification approach with optimal generalisation performance: support vector machines. Chemom. Intell. Lab. Syst. 2002;64:15–25. doi: 10.1016/S0169-7439(02)00046-1. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

