Economic and Clinical Burden of Managing Sickle Cell Disease with Recurrent Vaso-Occlusive Crises in the United States
- PMID: 37332020
- PMCID: PMC10329958
- DOI: 10.1007/s12325-023-02545-7
Economic and Clinical Burden of Managing Sickle Cell Disease with Recurrent Vaso-Occlusive Crises in the United States
Erratum in
-
Correction to: Economic and Clinical Burden of Managing Sickle Cell Disease with Recurrent Vaso-Occlusive Crises in the United States.Adv Ther. 2023 Nov;40(11):5130. doi: 10.1007/s12325-023-02629-4. Adv Ther. 2023. PMID: 37695467 Free PMC article. No abstract available.
Abstract
Introduction: The aim of this study was to describe the clinical complications, treatment use, healthcare resource utilization (HCRU), and costs among patients with sickle cell disease (SCD) with recurrent vaso-occlusive crises (VOCs) in the US.
Methods: Merative MarketScan Databases were used to identify patients with SCD with recurrent VOCs from March 1, 2010, to March 1, 2019. Inclusion criteria were ≥ 1 inpatient or ≥ 2 outpatient claims for SCD and ≥ 2 VOCs per year in any 2 consecutive years after the first qualifying SCD diagnosis. Individuals without SCD in these databases were used as matched controls. Patients were followed for ≥ 12 months, from their second VOC in the 2nd year (index date) to the earliest of inpatient death, end of continuous enrollment in medical/pharmacy benefits, or March 1, 2020. Outcomes were assessed during follow-up.
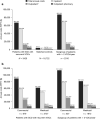
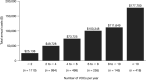
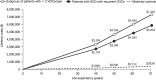
Results: In total, 3420 patients with SCD with recurrent VOCs and 16,722 matched controls were identified. Patients with SCD with recurrent VOCs had a mean of 5.0 VOCs (standard deviation [SD] = 6.0), 2.7 inpatient admissions (SD 2.9), and 5.0 emergency department visits (SD 8.0) per patient per year during follow-up. Compared to matched controls, patients with SCD with recurrent VOCs incurred higher annual ($67,282 vs. $4134) and lifetime ($3.8 million vs. $229,000 over 50 years) healthcare costs.
Conclusion: Patients with SCD with recurrent VOCs experience substantial clinical and economic burden driven by inpatient costs and frequent VOCs. There is a major unmet need for treatments that alleviate or eliminate clinical complications, including VOCs, and reduce healthcare costs in this patient population.
Keywords: Clinical decision-making; Costs and cost analysis; Healthcare costs; Healthcare economics and organizations; Medicaid; Sickle cell disease; Vaso-occlusive crisis.
© 2023. The Author(s).
Conflict of interest statement
Chuka Udeze, Yoojung Yang, and Nanxin Li are employees of Vertex Pharmaceuticals Incorporated and may hold stock/stock options. Urvi Mujumdar is a former employee of Vertex Pharmaceuticals Incorporated and may hold stock/stock options. Kristin A. Evans and Timothy Lillehaugen are employees of Merative and may hold stock/stock options. Janna Manjelievskaia was an employee of Merative at the time of this analysis and is now employed by Veradigm. Biree Andemarium has received research funding from the American Society of Hematology, Connecticut Department of Public Health, Forma Therapeutics, Global Blood Therapeutics, Hemanext, HRSA, Imara, Novartis, and PCORI, and has served as an advisory board member or consultant for Agios, Aruvant, Bayer, bluebird bio, CRISPR Therapeutics AG, CVS/Accordant, Cyclerion, Emmaus, Forma Therapeutics, GBT, Genentech, Hemanext, Novartis, NovoNordisk, Roche, Sanofi, TerSera, Terumo, and Vertex Pharmaceuticals Incorporated.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

