Exploring skeletal muscle tolerance and whole-body metabolic effects of FDA-approved drugs in a volumetric muscle loss model
- PMID: 37332022
- PMCID: PMC10277213
- DOI: 10.14814/phy2.15756
Exploring skeletal muscle tolerance and whole-body metabolic effects of FDA-approved drugs in a volumetric muscle loss model
Abstract
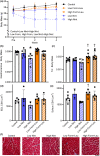
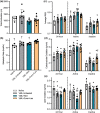
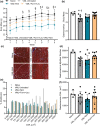
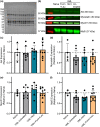
Volumetric muscle loss (VML) is associated with persistent functional impairment due to a lack of de novo muscle regeneration. As mechanisms driving the lack of regeneration continue to be established, adjunctive pharmaceuticals to address the pathophysiology of the remaining muscle may offer partial remediation. Studies were designed to evaluate the tolerance and efficacy of two FDA-approved pharmaceutical modalities to address the pathophysiology of the remaining muscle tissue after VML injury: (1) nintedanib (an anti-fibrotic) and (2) combined formoterol and leucine (myogenic promoters). Tolerance was first established by testing low- and high-dosage effects on uninjured skeletal muscle mass and myofiber cross-sectional area in adult male C57BL/6J mice. Next, tolerated doses of the two pharmaceutical modalities were tested in VML-injured adult male C57BL/6J mice after an 8-week treatment period for their ability to modulate muscle strength and whole-body metabolism. The most salient findings indicate that formoterol plus leucine mitigated the loss in muscle mass, myofiber number, whole-body lipid oxidation, and muscle strength, and resulted in a higher whole-body metabolic rate (p ≤ 0.016); nintedanib did not exacerbate or correct aspects of the muscle pathophysiology after VML. This supports ongoing optimization efforts, including scale-up evaluations of formoterol treatment in large animal models of VML.
Keywords: formoterol; muscle function; neuromusculoskeletal injury; nintedanib; skeletal muscle injury.
© 2023 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures
References
-
- Anthony, J. C. , Yoshizawa, F. , Anthony, T. G. , Vary, T. C. , Jefferson, L. S. , & Kimball, S. R. (2000). Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin‐sensitive pathway. The Journal of Nutrition, 130, 2413–2419. - PubMed
-
- Bedair, H. S. , Karthikeyan, T. , Quintero, A. , Li, Y. , & Huard, J. (2008). Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. The American Journal of Sports Medicine, 36, 1548–1554. - PubMed
-
- Buchholz, A. C. , Rafii, M. , & Pencharz, P. B. (2001). Is resting metabolic rate different between men and women? The British Journal of Nutrition, 86, 641–646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

