Pharmacokinetic analysis of vilobelimab, anaphylatoxin C5a and antidrug antibodies in PANAMO: a phase 3 study in critically ill, invasively mechanically ventilated COVID-19 patients
- PMID: 37332066
- PMCID: PMC10277268
- DOI: 10.1186/s40635-023-00520-8
Pharmacokinetic analysis of vilobelimab, anaphylatoxin C5a and antidrug antibodies in PANAMO: a phase 3 study in critically ill, invasively mechanically ventilated COVID-19 patients
Abstract
Background: Vilobelimab, a complement 5a (C5a)-specific monoclonal antibody, reduced mortality in critically ill COVID-19 patients in a phase 3 multicentre, randomized, double-blind, placebo-controlled study. As part of the study, vilobelimab concentrations and C5a levels as well as antidrug antibodies (ADAs) to vilobelimab were analysed.
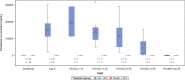
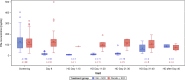
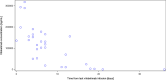
Results: From Oct 1, 2020 to Oct 4, 2021, 368 invasively mechanically ventilated COVID-19 patients were randomized: 177 patients were randomly assigned to receive vilobelimab while 191 patients received placebo. Pharmacokinetic sampling was only performed at sites in Western Europe. Blood samples for vilobelimab measurements were available for 93 of 177 (53%) patients in the vilobelimab group and 99 of 191 (52%) patients in the placebo group. On day 8, after three infusions, mean vilobelimab (trough) concentrations ranged from 21,799.3 to 302,972.1 ng/mL (geometric mean 137,881.3 ng/mL). Blood samples for C5a measurements were available for 94 of 177 (53%) patients in the vilobelimab group and 99 of 191 (52%) patients in the placebo group. At screening, C5a levels were highly elevated and comparable between groups. In the vilobelimab group, median C5a levels were 118.3 ng/mL [IQR 71.2-168.2 ng/mL] and in the placebo group, median C5a levels were 104.6 ng/mL [IQR 77.5-156.6 ng/mL]. By day 8, median C5a levels were reduced by 87% in the vilobelimab group (median 14.5 ng/mL [IQR 9.5-21.0 ng/mL], p < 0.001) versus an 11% increase in the placebo group (median 119.2 ng/mL [IQR 85.9-152.1 ng/mL]). Beyond day 8, though plasma sampling was sparse, C5a levels did not reach screening levels in the vilobelimab group while C5a levels remained elevated in the placebo group. Treatment-emergent ADAs were observed in one patient in the vilobelimab group at hospital discharge on day 40 and in one patient in the placebo group at hospital discharge on day 25.
Conclusions: This analysis shows that vilobelimab efficiently inhibits C5a in critically ill COVID-19 patients. There was no evidence of immunogenicity associated with vilobelimab treatment. Trial registration ClinicalTrials.gov, NCT04333420. Registered 3 April 2020, https://clinicaltrials.gov/ct2/show/NCT04333420.
Keywords: ADA; Antidrug antibodies; C5a; COVID-19; Complement; PK; Pharmacokinetic; RCT; SARS-CoV-2; Vilobelimab.
© 2023. The Author(s).
Conflict of interest statement
APJV received consulting fees from InflaRx for advisory work, paid to Amsterdam UMC. SR is an employee of Metronomia Clinical Research and a contracted statistical service provider for InflaRx. CT, MH, BPB and JD are employees of InflaRx and may hold shares and/or stock options in InflaRx. RG and NCR are founders, active officers, and executive directors of the board, and hold shares and/or stock options in InflaRx. All other authors declared no competing interests for this work.
Figures

References
-
- Vlaar APJ, Witzenrath M, van Paassen P, Heunks LMA, Mourvillier B, de Bruin S, et al. Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(12):1137–1146. doi: 10.1016/S2213-2600(22)00297-1. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

