Crystalline Nanoparticles of Water-Soluble Covalent Basket Cages (CBCs) for Encapsulation of Anticancer Drugs
- PMID: 37332078
- PMCID: PMC10528532
- DOI: 10.1002/anie.202306722
Crystalline Nanoparticles of Water-Soluble Covalent Basket Cages (CBCs) for Encapsulation of Anticancer Drugs
Abstract
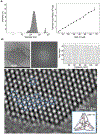
We herein describe the preparation, assembly, recognition characteristics, and biocompatibility of novel covalent basket cage CBC-11, composed of four molecular baskets linked to four trivalent aromatic amines through amide groups. The cage is tetrahedral in shape and similar in size to small proteins (Mw =8637 g/mol) with a spacious nonpolar interior for accommodating multiple guests. While 24 carboxylates at the outer surface of CBC-11 render it soluble in aqueous phosphate buffer (PBS) at pH=7.0, the amphiphilic nature prompts its assembly into nanoparticles (d=250 nm, DLS). Cryo-TEM examination of nanoparticles revealed their crystalline nature with wafer-like shapes and hexagonally arranged cages. Nanoparticulate CBC-11 traps anticancer drugs irinotecan and doxorubicin, with each cage binding up to four drug molecules in a non-cooperative manner. The inclusion complexation resulted in nanoparticles growing in size and precipitating. In media containing mammalian cells (HCT 116, human colon carcinoma), the IC50 value of CBC-11 was above 100 μM. While this work presents the first example of a large covalent organic cage operating in water at the physiological pH and forming crystalline nanoparticles, it also demonstrates its biocompatibility and potential to act as a polyvalent binder of drugs for their sequestration or delivery.
Keywords: Anticancer Drugs; Covalent Organic Cages; Drug Delivery; Host-Guest Chemistry; Sequestration.
© 2023 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Figures



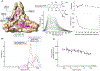

Similar articles
-
Water-Soluble Molecular Cages for Biological Applications.Molecules. 2024 Apr 4;29(7):1621. doi: 10.3390/molecules29071621. Molecules. 2024. PMID: 38611902 Free PMC article. Review.
-
Comparing organic and metallo-organic hydrazone molecular cages as potential carriers for doxorubicin delivery.Chem Sci. 2024 Jun 7;15(26):10010-10017. doi: 10.1039/d4sc02294g. eCollection 2024 Jul 3. Chem Sci. 2024. PMID: 38966373 Free PMC article.
-
Dissipative Formation of Covalent Basket Cages.Angew Chem Int Ed Engl. 2022 Aug 15;61(33):e202207418. doi: 10.1002/anie.202207418. Epub 2022 Jul 11. Angew Chem Int Ed Engl. 2022. PMID: 35723284 Free PMC article.
-
Light-Triggered Transformation of Molecular Baskets into Organic Nanoparticles.Chemistry. 2019 Jan 2;25(1):273-279. doi: 10.1002/chem.201803693. Epub 2018 Oct 12. Chemistry. 2019. PMID: 30133001
-
Assembly of Protein Cages for Drug Delivery.Pharmaceutics. 2022 Nov 26;14(12):2609. doi: 10.3390/pharmaceutics14122609. Pharmaceutics. 2022. PMID: 36559102 Free PMC article. Review.
Cited by
-
Charge-assisted hydrogen bonding in a bicyclic amide cage: an effective approach to anion recognition and catalysis in water.Chem Sci. 2024 Sep 18;15(39):16040-9. doi: 10.1039/d4sc05236f. Online ahead of print. Chem Sci. 2024. PMID: 39309075 Free PMC article.
-
Water-Soluble Molecular Cages for Biological Applications.Molecules. 2024 Apr 4;29(7):1621. doi: 10.3390/molecules29071621. Molecules. 2024. PMID: 38611902 Free PMC article. Review.
-
Fluoride Enhances Alcohol Binding Within a Trigonal-Prismatic Metal-Organic Capsule.Angew Chem Int Ed Engl. 2025 Jul;64(29):e202505137. doi: 10.1002/anie.202505137. Epub 2025 May 19. Angew Chem Int Ed Engl. 2025. PMID: 40344164 Free PMC article.
-
Molecular bowls for inclusion complexation of toxic anticancer drug methotrexate.Chem Sci. 2024 May 28;15(26):10155-10163. doi: 10.1039/d3sc05627a. eCollection 2024 Jul 3. Chem Sci. 2024. PMID: 38966368 Free PMC article.
-
Comparing organic and metallo-organic hydrazone molecular cages as potential carriers for doxorubicin delivery.Chem Sci. 2024 Jun 7;15(26):10010-10017. doi: 10.1039/d4sc02294g. eCollection 2024 Jul 3. Chem Sci. 2024. PMID: 38966373 Free PMC article.
References
-
- Zhang G, Mastalerz M, Chem. Soc. Rev 2014, 43, 1934. - PubMed
-
- Brotin T, Dutasta J-P, Chem. Rev 2009, 109, 88. - PubMed
-
- Gabard J, Collet A, J. Chem. Soc., Chem. Commun 1981, 1137;
- Cram DJ, Karbach S, Kim YH, Baczynskyj L, Kallemeyn GW, J. Am. Chem. Soc 1985, 107, 2575.
-
- Cram DJ, Science 1983, 219, 1177. - PubMed
-
- Galan A, Ballester P, Chem. Soc. Rev 2016, 45, 1720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

