A PDGFRB- and CD40-targeting bispecific AffiMab induces stroma-targeted immune cell activation
- PMID: 37332119
- PMCID: PMC10332328
- DOI: 10.1080/19420862.2023.2223750
A PDGFRB- and CD40-targeting bispecific AffiMab induces stroma-targeted immune cell activation
Abstract
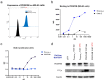
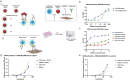
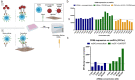
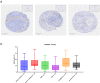
CD40 agonism by systemic administration of CD40 monoclonal antibodies has been explored in clinical trials for immunotherapy of cancer, uncovering enormous potential, but also dosing challenges in terms of systemic toxicity. CD40-dependent activation of antigen presenting cells is dependent on crosslinking of the CD40 receptor. Here we exploited this requisite by coupling crosslinking to cancer-receptor density by dual-targeting of CD40 and platelet-derived growth factor receptor beta (PDGFRB), which is highly expressed in the stroma of various types of tumors. A novel PDGFRBxCD40 Fc-silenced bispecific AffiMab was developed to this end to test whether it is possible to activate CD40 in a PDGFRB-targeted manner. A PDGFRB-binding Affibody molecule was fused to each heavy chain of an Fc-silenced CD40 agonistic monoclonal antibody to obtain a bispecific "AffiMab". Binding of the AffiMab to both PDGFRB and CD40 was confirmed by surface plasmon resonance, bio-layer interferometry and flow cytometry, through analysis of cells expressing respective target. In a reporter assay, the AffiMab displayed increased CD40 potency in the presence of PDGFRB-conjugated beads, in a manner dependent on PDGFRB amount/bead. To test the concept in immunologically relevant systems with physiological levels of CD40 expression, the AffiMab was tested in human monocyte-derived dendritic cells (moDCs) and B cells. Expression of activation markers was increased in moDCs specifically in the presence of PDGFRB-conjugated beads upon AffiMab treatment, while the Fc-silenced CD40 mAb did not stimulate CD40 activation. As expected, the AffiMab did not activate moDCs in the presence of unconjugated beads. Finally, in a co-culture experiment, the AffiMab activated moDCs and B cells in the presence of PDGFRB-expressing cells, but not in co-cultures with PDGFRB-negative cells. Collectively, these results suggest the possibility to activate CD40 in a PDGFRB-targeted manner in vitro. This encourages further investigation and the development of such an approach for the treatment of solid cancers.
Keywords: CD40; Cancer; PDGFRB; immuno-oncology; microenvironment.
Conflict of interest statement
A. Mega, E. Ryer, A. Sköld, A. Sandegren, E. Backström Rydin and F.Y. Frejd are employed at Affibody AB. A. Mebrahtu, G. Aniander, J. Rockberg and A. Östman have no conflicts of interest that are directly relevant to the content of this article.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
