Vasculature in the mouse colon and spatial relationships with the enteric nervous system, glia, and immune cells
- PMID: 37332321
- PMCID: PMC10272736
- DOI: 10.3389/fnana.2023.1130169
Vasculature in the mouse colon and spatial relationships with the enteric nervous system, glia, and immune cells
Abstract
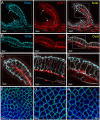
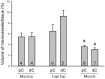
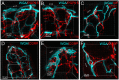
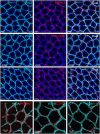
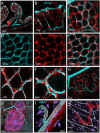
The distribution, morphology, and innervation of vasculature in different mouse colonic segments and layers, as well as spatial relationships of the vasculature with the enteric plexuses, glia, and macrophages are far from being complete. The vessels in the adult mouse colon were stained by the cardiovascular perfusion of wheat germ agglutinin (WGA)-Alexa Fluor 448 and by CD31 immunoreactivity. Nerve fibers, enteric glia, and macrophages were immunostained in the WGA-perfused colon. The blood vessels entered from the mesentery to the submucosa and branched into the capillary networks in the mucosa and muscularis externa. The capillary net formed anastomosed rings at the orifices of mucosa crypts, and the capillary rings surrounded the crypts individually in the proximal colon and more than two crypts in the distal colon. Microvessels in the muscularis externa with myenteric plexus were less dense than in the mucosa and formed loops. In the circular smooth muscle layer, microvessels were distributed in the proximal, but not the distal colon. Capillaries did not enter the enteric ganglia. There were no significant differences in microvascular volume per tissue volume between the proximal and distal colon either in the mucosa or muscularis externa containing the myenteric plexus. PGP9.5-, tyrosine hydroxylase-, and calcitonin gene-related peptide (CGRP)-immunoreactive nerve fibers were distributed along the vessels in the submucosa. In the mucosa, PGP9.5-, CGRP-, and vasoactive intestinal peptide (VIP)-immunoreactive nerves terminated close to the capillary rings, while cells and processes labeled by S100B and glial fibrillary acidic protein were distributed mainly in the lamina propria and lower portion of the mucosa. Dense Iba1 immunoreactive macrophages were closely adjacent to the mucosal capillary rings. There were a few macrophages, but no glia in apposition to microvessels in the submucosa and muscularis externa. In conclusion, in the mouse colon, (1) the differences in vasculature between the proximal and distal colon were associated with the morphology, but not the microvascular amount per tissue volume in the mucosa and muscle layers; (2) the colonic mucosa contained significantly more microvessels than the muscularis externa; and (3) there were more CGRP and VIP nerve fibers found close to microvessels in the mucosa and submucosa than in the muscle layers.
Keywords: colon; immunohistochemistry; microvessel; mouse; vasculature; wheat germ agglutinin.
Copyright © 2023 Wang, Yuan and Taché.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

