Axonal mapping of the motor cranial nerves
- PMID: 37332322
- PMCID: PMC10272770
- DOI: 10.3389/fnana.2023.1198042
Axonal mapping of the motor cranial nerves
Abstract
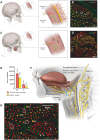
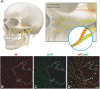
Basic behaviors, such as swallowing, speech, and emotional expressions are the result of a highly coordinated interplay between multiple muscles of the head. Control mechanisms of such highly tuned movements remain poorly understood. Here, we investigated the neural components responsible for motor control of the facial, masticatory, and tongue muscles in humans using specific molecular markers (ChAT, MBP, NF, TH). Our findings showed that a higher number of motor axonal population is responsible for facial expressions and tongue movements, compared to muscles in the upper extremity. Sensory axons appear to be responsible for neural feedback from cutaneous mechanoreceptors to control the movement of facial muscles and the tongue. The newly discovered sympathetic axonal population in the facial nerve is hypothesized to be responsible for involuntary control of the muscle tone. These findings shed light on the pivotal role of high efferent input and rich somatosensory feedback in neuromuscular control of finely adjusted cranial systems.
Keywords: facial muscles; facial nerve; hypoglossal nerve; masseteric nerve; motor control; proprioception; sensory feedback; sympathetic axons.
Copyright © 2023 Tereshenko, Maierhofer, Dotzauer, Laengle, Politikou, Carrero Rojas, Festin, Luft, Jaklin, Hruby, Gohritz, Farina, Blumer, Bergmeister and Aszmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Angelov D. N. (2016). Embryology and anatomy of the facial nerve: Correlates of misdirected reinnervation and poor recovery of function after lesions. Facial Nerve Disord. Dis. Diag. Manage. 1, 8–10. 10.1055/b-0036-140477 - DOI
-
- Baumel J. (1974). Trigeminal-facial nerve communcations. Arch. Otolaryngol. 99 34–44. - PubMed
-
- Borschel G. H., Kawamura D. H., Kasukurthi R., Hunter D. A., Zuker R. M., Woo A. S. (2012). The motor nerve to the masseter muscle: An anatomic and histomorphometric study to facilitate its use in facial reanimation. J. Plastic Reconstruc. Aesthetic Surg. 65 363–366. 10.1016/j.bjps.2011.09.026 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

