Elexacaftor-tezacaftor-ivacaftor in patients with cystic fibrosis ineligible for clinical trials: a 24-week observational study
- PMID: 37332357
- PMCID: PMC10275572
- DOI: 10.3389/fphar.2023.1178009
Elexacaftor-tezacaftor-ivacaftor in patients with cystic fibrosis ineligible for clinical trials: a 24-week observational study
Abstract
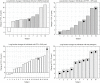
Introduction: Seminal clinical trials with the triple combination of elexacaftor-tezacaftor-ivacaftor (ETI) demonstrated clinical efficacy in people with cystic fibrosis (pwCF) who carry at least one F508del mutation. However, due to exclusion criteria of these clinical trials, the effect of ETI was not studied in a substantial number of pwCF. Thus, we ran a single center trial to evaluate a clinical efficacy of ETI treatment in adult pwCF who were ineligible for enrollment in registration studies. Methods: PwCF on ETI with prior lumacaftor-ivacaftor therapy, severe airway obstruction, well-preserved lung function, or with airway infection with pathogens at risk of more rapid decline in lung function formed the study group, while all the others on ETI formed the control group. Lung function, nutritional status and sweat chloride concentration were assessed before and after initialization of ETI therapy over a 6-month period. Results: Approximately a half of the ETI-treated pwCF at the adult Prague CF center (49 of 96) were assigned to the study group. Their mean changes in body mass index ( + 1.04 kg/m2) and in sweat chloride concentration (-48.4 mmol/L) were similar to the control group ( + 1.02 kg/m2; -49.7 mmol/L), while the mean change in percent predicted forced expiratory volume in 1 s (ppFEV1; + 10.3 points) was significantly lower than in the control group ( + 15.8 points) (p = 0.0015). In the subgroup analysis, pwCF with severe airway obstruction (ppFEV1 <40) and pwCF with well-preserved lung function (ppFEV1 >90) showed a less potential for improvement in lung function during the ETI treatment than controls (median change in ppFEV1 + 4.9 points and + 9.5 points, respectively). Conclusion: PwCF not eligible for inclusion in clinical trials demonstrated improvement in lung function and nutritional status following the initiation of treatment with the ETI combination. Moderate increase in ppFEV1 was observed in those with severe airway obstruction or well-preserved lung function.
Keywords: cystic fibrosis; elexacaftor-tezacaftor-ivacaftor; lung function; nutritional status; sweat chloride concentration; variant specific therapy.
Copyright © 2023 Fila, Grandcourtova, Bilkova and Drevinek.
Conflict of interest statement
LF and PD recived speakers fee and advisory boards members fee from Vertex Pharmaceuticals, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Burgel P. R., Durieu I., Chiron R., Ramel S., Danner-Boucher I., Prevotat A., et al. (2021). Rapid improvement after starting elexacaftor-tezacaftor-ivacaftor in patients with cystic fibrosis and advanced pulmonary disease. Am. J. Respir. Crit. Care Med. 204 (1), 64–73. 10.1164/rccm.202011-4153OC - DOI - PubMed
-
- Cystic Fibrosis Foundation (2022). Cystic fibrosis foundation patient registry 2021 annual data report. Bethesda, Maryland: ©2022 Cystic Fibrosis Foundation. Available at: https://www.cff.org/medical-professionals/patient-registry (Accessed February 20, 2023).
LinkOut - more resources
Full Text Sources
Miscellaneous

