ESHRE good practice recommendations on recurrent implantation failure
- PMID: 37332387
- PMCID: PMC10270320
- DOI: 10.1093/hropen/hoad023
ESHRE good practice recommendations on recurrent implantation failure
Abstract
Study question: How should recurrent implantation failure (RIF) in patients undergoing ART be defined and managed?
Summary answer: This is the first ESHRE good practice recommendations paper providing a definition for RIF together with recommendations on how to investigate causes and contributing factors, and how to improve the chances of a pregnancy.
What is known already: RIF is a challenge in the ART clinic, with a multitude of investigations and interventions offered and applied in clinical practice, often without biological rationale or with unequivocal evidence of benefit.
Study design size duration: This document was developed according to a predefined methodology for ESHRE good practice recommendations. Recommendations are supported by data from the literature, if available, and the results of a previously published survey on clinical practice in RIF and the expertise of the working group. A literature search was performed in PubMed and Cochrane focussing on 'recurrent reproductive failure', 'recurrent implantation failure', and 'repeated implantation failure'.
Participants/materials setting methods: The ESHRE Working Group on Recurrent Implantation Failure included eight members representing the ESHRE Special Interest Groups for Implantation and Early Pregnancy, Reproductive Endocrinology, and Embryology, with an independent chair and an expert in statistics. The recommendations for clinical practice were formulated based on the expert opinion of the working group, while taking into consideration the published data and results of the survey on uptake in clinical practice. The draft document was then open to ESHRE members for online peer review and was revised in light of the comments received.
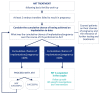
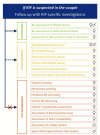
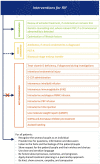
Main results and the role of chance: The working group recommends considering RIF as a secondary phenomenon of ART, as it can only be observed in patients undergoing IVF, and that the following description of RIF be adopted: 'RIF describes the scenario in which the transfer of embryos considered to be viable has failed to result in a positive pregnancy test sufficiently often in a specific patient to warrant consideration of further investigations and/or interventions'. It was agreed that the recommended threshold for the cumulative predicted chance of implantation to identify RIF for the purposes of initiating further investigation is 60%. When a couple have not had a successful implantation by a certain number of embryo transfers and the cumulative predicted chance of implantation associated with that number is greater than 60%, then they should be counselled on further investigation and/or treatment options. This term defines clinical RIF for which further actions should be considered. Nineteen recommendations were formulated on investigations when RIF is suspected, and 13 on interventions. Recommendations were colour-coded based on whether the investigations/interventions were recommended (green), to be considered (orange), or not recommended, i.e. not to be offered routinely (red).
Limitations reasons for caution: While awaiting the results of further studies and trials, the ESHRE Working Group on Recurrent Implantation Failure recommends identifying RIF based on the chance of successful implantation for the individual patient or couple and to restrict investigations and treatments to those supported by a clear rationale and data indicating their likely benefit.
Wider implications of the findings: This article provides not only good practice advice but also highlights the investigations and interventions that need further research. This research, when well-conducted, will be key to making progress in the clinical management of RIF.
Study funding/competing interests: The meetings and technical support for this project were funded by ESHRE. N.M. declared consulting fees from ArtPRED (The Netherlands) and Freya Biosciences (Denmark); Honoraria for lectures from Gedeon Richter, Merck, Abbott, and IBSA; being co-founder of Verso Biosense. He is Co-Chief Editor of Reproductive Biomedicine Online (RBMO). D.C. declared being an Associate Editor of Human Reproduction Update, and declared honoraria for lectures from Merck, Organon, IBSA, and Fairtility; support for attending meetings from Cooper Surgical, Fujifilm Irvine Scientific. G.G. declared that he or his institution received financial or non-financial support for research, lectures, workshops, advisory roles, or travelling from Ferring, Merck, Gedeon-Richter, PregLem, Abbott, Vifor, Organon, MSD, Coopersurgical, ObsEVA, and ReprodWissen. He is an Editor of the journals Archives of Obstetrics and Gynecology and Reproductive Biomedicine Online, and Editor in Chief of Journal Gynäkologische Endokrinologie. He is involved in guideline developments and quality control on national and international level. G.L. declared he or his institution received honoraria for lectures from Merck, Ferring, Vianex/Organon, and MSD. He is an Associate Editor of Human Reproduction Update, immediate past Coordinator of Special Interest Group for Reproductive Endocrinology of ESHRE and has been involved in Guideline Development Groups of ESHRE and national fertility authorities. D.J.M. declared being an Associate Editor for Human Reproduction Open and statistical Advisor for Reproductive Biomedicine Online. B.T. declared being shareholder of Reprognostics and she or her institution received financial or non-financial support for research, clinical trials, lectures, workshops, advisory roles or travelling from support for attending meetings from Ferring, MSD, Exeltis, Merck Serono, Bayer, Teva, Theramex and Novartis, Astropharm, Ferring. The other authors had nothing to disclose.
Disclaimer: This Good Practice Recommendations (GPR) document represents the views of ESHRE, which are the result of consensus between the relevant ESHRE stakeholders and are based on the scientific evidence available at the time of preparation. ESHRE GPRs should be used for information and educational purposes. They should not be interpreted as setting a standard of care or be deemed inclusive of all proper methods of care, or be exclusive of other methods of care reasonably directed to obtaining the same results. They do not replace the need for application of clinical judgement to each individual presentation, or variations based on locality and facility type. Furthermore, ESHRE GPRs do not constitute or imply the endorsement, or favouring, of any of the included technologies by ESHRE.
Keywords: ART; ESHRE; IVF failure; embryo transfer; good practice; guidelines; implantation failure; recurrent implantation failure.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
N.M. declared consulting fees from ArtPRED (The Netherlands) and Freya Biosciences (Denmark); honoraria for lectures from Gedeon Richter, Merck, Abbott, and IBSA; being co-founder of Verso Biosense. He is Co-Chief Editor of Reproductive Biomedicine Online (RBMO). D.C. declared being an Associate Editor of Human Reproduction Update, and declared honoraria for lectures from Merck, Organon, IBSA, and Fairtility; support for attending meetings from Cooper Surgical, Fujifilm Irvine Scientific. G.G. declared that he or his institution received financial or non-financial support for research, lectures, workshops, advisory roles or travelling from Ferring, Merck, Gedeon-Richter, PregLem, Abbott, Vifor, Organon, MSD, Coopersurgical, ObsEVA, and ReprodWissen. He is an Editor of the journals Archives of Obstetrics and Gynecology and Reproductive Biomedicine Online, and Editor in Chief of Journal Gynäkologische Endokrinologie. He is involved in guideline developments and quality control on national and international level. G.L. declared he or his institution received honoraria for lectures from Merck, Ferring, Vianex/Organon, and MSD. He is an Associate Editor of Human Reproduction Update, immediate past Co-ordinator of Special Interest Group for Reproductive Endocrinology of ESHRE and has been involved in Guideline Development Groups of ESHRE and national fertility authorities. D.J.M. declared being an Associate Editor for Human Reproduction Open and statistical Advisor for Reproductive Biomedicine Online. B.T. declared being shareholder of Reprognostics and she or her institution received financial or non-financial support for research, clinical trials, lectures, workshops, advisory roles or travelling from support for attending meetings from Ferring, MSD, Exeltis, Merck Serono, Bayer, Teva, Theramex and Novartis, Astropharm, Ferring. The other authors had nothing to disclose.
Figures

References
-
- Abdolmohammadi-Vahid S, Pashazadeh F, Pourmoghaddam Z, Aghebati-Maleki L, Abdollahi-Fard S, Yousefi M.. The effectiveness of IVIG therapy in pregnancy and live birth rate of women with recurrent implantation failure (RIF): a systematic review and meta-analysis. J Reprod Immunol 2019;134–135:28–33. - PubMed
-
- ACOG. Infertility workup for the women’s health specialist: ACOG Committee Opinion, Number 781. Obstet Gynecol 2019;133:e377–e384. - PubMed
-
- Alecsandru D, Barrio A, Garrido N, Aparicio P, Pellicer A, Moffett A, García-Velasco JA.. Parental human leukocyte antigen-C allotypes are predictive of live birth rate and risk of poor placentation in assisted reproductive treatment. Fertil Steril 2020;114:809–817. - PubMed
-
- Ali SB, Jeelall Y, Pennell CE, Hart R, McLean-Tooke A, Lucas M.. The role of immunological testing and intervention in reproductive medicine: a fertile collaboration? Am J Reprod Immunol 2018;79:e12784. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
