Efficacy of ripasudil in reducing intraocular pressure and medication score for ocular hypertension with inflammation and corticosteroid
- PMID: 37332549
- PMCID: PMC10250944
- DOI: 10.18240/ijo.2023.06.11
Efficacy of ripasudil in reducing intraocular pressure and medication score for ocular hypertension with inflammation and corticosteroid
Abstract
Aim: To investigate the efficacy of ripasudil, a Rho kinase inhibitor, in reducing intraocular pressure (IOP) and medication scores of anti-glaucoma drugs in patients with ocular hypertension with inflammation and corticosteroid.
Methods: The study included 11 patients diagnosed with ocular hypertension with inflammation and corticosteroid, all of whom were prescribed ripasudil eye drops and followed up for at least 2y after the initiation of treatment. IOP was measured using a non-contact tonometer before enrollment and at each follow-up visit. The medication score of glaucoma eye drops was calculated for each patient.
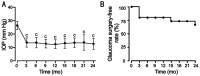
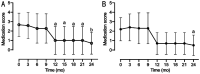
Results: The mean IOP (26.4±2.9 mm Hg before treatment) significantly decreased after ripasudil therapy (13.7±3.3 mm Hg at 3mo) and remained stable in the low-teens during the 2-year follow-up period (P<0.0001). A significant decrease in the medication score was observed at 12mo or later after the initiation of ripasudil therapy (P<0.05). Both baseline medication scores and glaucomatous optic disc change rates were significantly higher in the five eyes that required glaucoma surgery during the 2-year observation period than the 10 eyes that did not require surgery.
Conclusion: Our results demonstrate the efficacy of ripasudil, in reducing IOP and the medication score over a 2-year treatment period in patients with ocular hypertension with inflammation and corticosteroid. Our findings also suggest that ripasudil could reduce the IOP in uveitic glaucoma patients with both lower baseline medication score and lower glaucomatous optic disc change rate.
Keywords: Rho kinase inhibitor; glaucoma; intraocular pressure; medication score; uveitis.
International Journal of Ophthalmology Press.
Figures


Similar articles
-
Efficacy and safety of ripasudil, a Rho-associated kinase inhibitor, in eyes with uveitic glaucoma.Graefes Arch Clin Exp Ophthalmol. 2018 Apr;256(4):809-814. doi: 10.1007/s00417-018-3933-9. Epub 2018 Feb 21. Graefes Arch Clin Exp Ophthalmol. 2018. PMID: 29468405
-
Safety and efficacy of ripasudil in Japanese patients with glaucoma or ocular hypertension: 12-month interim analysis of ROCK-J, a post-marketing surveillance study.BMC Ophthalmol. 2020 Jul 9;20(1):275. doi: 10.1186/s12886-020-01490-1. BMC Ophthalmol. 2020. PMID: 32646383 Free PMC article.
-
Intra-ocular pressure-lowering effects of a Rho kinase inhibitor, ripasudil (K-115), over 24 hours in primary open-angle glaucoma and ocular hypertension: a randomized, open-label, crossover study.Acta Ophthalmol. 2015 Jun;93(4):e254-60. doi: 10.1111/aos.12599. Epub 2014 Dec 9. Acta Ophthalmol. 2015. PMID: 25487877 Clinical Trial.
-
Ripasudil hydrochloride hydrate: targeting Rho kinase in the treatment of glaucoma.Expert Opin Pharmacother. 2017 Oct;18(15):1669-1673. doi: 10.1080/14656566.2017.1378344. Epub 2017 Sep 14. Expert Opin Pharmacother. 2017. PMID: 28893104 Review.
-
Ripasudil Hydrochloride Hydrate in the Treatment of Glaucoma: Safety, Efficacy, and Patient Selection.Clin Ophthalmol. 2020 May 6;14:1229-1236. doi: 10.2147/OPTH.S216907. eCollection 2020. Clin Ophthalmol. 2020. PMID: 32440089 Free PMC article. Review.
Cited by
-
Lasamide Containing Sulfonylpiperazines as Effective Agents for the Management of Glaucoma Associated Symptoms.ChemMedChem. 2024 Dec 16;19(24):e202400601. doi: 10.1002/cmdc.202400601. Epub 2024 Nov 8. ChemMedChem. 2024. PMID: 39319579 Free PMC article.
-
Ripasudil as a Potential Therapeutic Agent in Treating Secondary Glaucoma in HTLV-1-Uveitis: An In Vitro Analysis.Int J Mol Sci. 2024 Mar 12;25(6):3229. doi: 10.3390/ijms25063229. Int J Mol Sci. 2024. PMID: 38542203 Free PMC article.
-
Update on Diagnosis and Treatment of Uveitic Glaucoma.J Clin Med. 2024 Feb 20;13(5):1185. doi: 10.3390/jcm13051185. J Clin Med. 2024. PMID: 38592059 Free PMC article. Review.
References
-
- Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Araie M. Phase 2 randomized clinical study of a rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2013;156(4):731–736.e2. - PubMed
-
- Kusuhara S, Katsuyama A, Matsumiya W, Nakamura M. Efficacy and safety of ripasudil, a Rho-associated kinase inhibitor, in eyes with uveitic glaucoma. Graefes Arch Clin Exp Ophthalmol. 2018;256(4):809–814. - PubMed
-
- Uchida T, Honjo M, Yamagishi R, Aihara M. The anti-inflammatory effect of ripasudil (K-115), a rho kinase (ROCK) inhibitor, on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2017;58(12):5584–5593. - PubMed
LinkOut - more resources
Full Text Sources
