Subconjunctival conbercept for the treatment of corneal neovascularization
- PMID: 37332556
- PMCID: PMC10250950
- DOI: 10.18240/ijo.2023.06.06
Subconjunctival conbercept for the treatment of corneal neovascularization
Abstract
Aim: To investigate the effectiveness and safety of subconjunctival injection of conbercept in the treatment of corneal neovascularization (CNV).
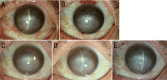
Methods: The data on 10 consecutively recruited patients with CNV who received a subconjunctival conbercept (1 mg) once, and measured the area, length, and diameter of neovascularization before and after (1d, 1, 2wk, and 1mo) treatment as well as the occurrence of systemic and ocular complications after treatment were analyzed.
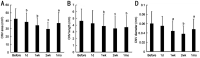
Results: There was a statistically significant reduction in the area of CNV one day after treatment (mean±SD: 38.46±11.36 mm2), compared with before treatment (42.46±12.80 mm2, P<0.01). There was also a statistically significant reduction in the length (3.86±1.80 mm vs 4.64±1.77 mm, P<0.01) and diameter (0.044±0.022 vs 0.060±0.026, P<0.05) of CNV, one week after treatment comparing to before treatment. The reduction in all three parameters was maximized at two weeks after treatment (area: 29.49±8.83 mm2, P<0.001; length: 3.50±1.88 mm, P<0.001; and diameter: 0.038±0.017 mm, P<0.01). No severe systemic or ocular complication was observed during the study.
Conclusion: During the observation period of one-month, subconjunctival injection of conbercept is an effective and safe method for the reduction of CNV. It may be effective as a preoperative drug for neovascular corneal transplantation.
Keywords: anti-vascular endothelial growth factor; conbercept; corneal neovascularization; subconjunctival injection.
International Journal of Ophthalmology Press.
Figures
Similar articles
-
Effect of Conbercept on Corneal Neovascularization in a Rabbit Model.Semin Ophthalmol. 2023 Oct;38(7):670-678. doi: 10.1080/08820538.2023.2201652. Epub 2023 Apr 14. Semin Ophthalmol. 2023. PMID: 37058000
-
Subconjunctival bevacizumab for corneal neovascularization.Acta Ophthalmol. 2010 Dec;88(8):868-71. doi: 10.1111/j.1755-3768.2009.01585.x. Acta Ophthalmol. 2010. PMID: 19519730
-
Corneal neovascularization inhibition and wound healing impregnability of conbercept on rabbit cornea after penetrating keratoplasty.Cutan Ocul Toxicol. 2022 Mar;41(1):98-104. doi: 10.1080/15569527.2022.2050745. Epub 2022 Apr 3. Cutan Ocul Toxicol. 2022. PMID: 35373690
-
Profile of conbercept in the treatment of neovascular age-related macular degeneration.Drug Des Devel Ther. 2015 Apr 22;9:2311-20. doi: 10.2147/DDDT.S67536. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25960634 Free PMC article. Review.
-
Updates on the Management of Ocular Vasculopathies with VEGF Inhibitor Conbercept.Curr Eye Res. 2020 Dec;45(12):1467-1476. doi: 10.1080/02713683.2020.1781193. Epub 2020 Jul 7. Curr Eye Res. 2020. PMID: 32631094 Review.
Cited by
-
Management of corneal neovascularization: Current and emerging therapeutic approaches.Indian J Ophthalmol. 2024 May 1;72(Suppl 3):S354-S371. doi: 10.4103/IJO.IJO_3043_23. Epub 2024 Apr 20. Indian J Ophthalmol. 2024. PMID: 38648452 Free PMC article. Review.
References
-
- Yin QC, Han HJ, Shi KX, Zhou JY, Zheng SF, Yao K, Shentu XC. Targeted dexamethasone nano-prodrug for corneal neovascularization management. Biomed J. 2023:S2319-S4170(23)00029-X. - PubMed
-
- Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 2011;56(2):95–113. - PubMed
-
- Yeung SN, Lichtinger A, Kim P, Amiran MD, Slomovic AR. Combined use of subconjunctival and intracorneal bevacizumab injection for corneal neovascularization. Cornea. 2011;30(10):1110–1114. - PubMed
LinkOut - more resources
Full Text Sources
