The septate junction component bark beetle is required for Drosophila intestinal barrier function and homeostasis
- PMID: 37332603
- PMCID: PMC10276166
- DOI: 10.1016/j.isci.2023.106901
The septate junction component bark beetle is required for Drosophila intestinal barrier function and homeostasis
Abstract
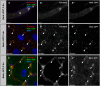
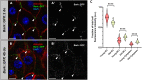
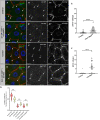
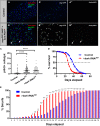
Age-related loss of intestinal barrier function has been documented across species, but the causes remain unknown. The intestinal barrier is maintained by tight junctions (TJs) in mammals and septate junctions (SJs) in insects. Specialized TJs/SJs, called tricellular junctions (TCJs), are located at the nexus of three adjacent cells, and we have shown that aging results in changes to TCJs in intestines of adult Drosophila melanogaster. We now demonstrate that localization of the TCJ protein bark beetle (Bark) decreases in aged flies. Depletion of bark from enterocytes in young flies led to hallmarks of intestinal aging and shortened lifespan, whereas depletion of bark in progenitor cells reduced Notch activity, biasing differentiation toward the secretory lineage. Our data implicate Bark in EC maturation and maintenance of intestinal barrier integrity. Understanding the assembly and maintenance of TCJs to ensure barrier integrity may lead to strategies to improve tissue integrity when function is compromised.
Keywords: Cell biology; Entomology; Molecular biology.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Marchiando A.M., Graham W.V., Turner J.R. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. 2010;5:119–144. - PubMed
-
- Kirkwood T.B.L. Intrinsic ageing of gut epithelial stem cells. Mech. Ageing Dev. 2004;125:911–915. - PubMed
-
- Ren W.y., Wu K.f., Li X., Luo M., Liu H.c., Zhang S.c., Hu Y. Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clin. Exp. Res. 2014;26:183–191. - PubMed
-
- Schiffrin E.J., Morley J.E., Donnet-Hughes A., Guigoz Y. The inflammatory status of the elderly: the intestinal contribution. Mutat. Res. 2010;690:50–56. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

