Amino acid permease RcAAP1 increases the uptake and phloem translocation of an L-valine-phenazine-1-carboxylic acid conjugate
- PMID: 37332709
- PMCID: PMC10272580
- DOI: 10.3389/fpls.2023.1191250
Amino acid permease RcAAP1 increases the uptake and phloem translocation of an L-valine-phenazine-1-carboxylic acid conjugate
Abstract
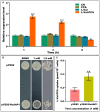
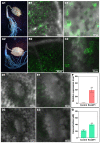
Amino acid conjugates of pesticides can promote the phloem translocation of parent ingredients, allowing for the reduction of usage, and decreased environmental pollution. Plant transporters play important roles in the uptake and phloem translocation of such amino acid-pesticide conjugates such as L-Val-PCA (L-valine-phenazine-1-carboxylic acid conjugate). However, the effects of an amino acid permease, RcAAP1, on the uptake and phloem mobility of L-Val-PCA are still unclear. Here, the relative expression levels of RcAAP1 were found to be up-regulated 2.7-fold and 2.2-fold by the qRT-PCR after L-Val-PCA treatments of Ricinus cotyledons for 1 h and 3 h, respectively. Subsequently, expression of RcAAP1 in yeast cells increased the L-Val-PCA uptake (0.36 μmol/107 cells), which was 2.1-fold higher than the control (0.17 μmol/107 cells). Pfam analysis suggested RcAAP1 with its 11 transmembrane domains belongs to the amino acid transporter family. Phylogenetic analysis found RcAAP1 to be strongly similar to AAP3 in nine other species. Subcellular localization showed that fusion RcAAP1-eGFP proteins were observed in the plasma membrane of mesophyll cells and phloem cells. Furthermore, overexpression of RcAAP1 for 72 h significantly increased the phloem mobility of L-Val-PCA in Ricinus seedlings, and phloem sap concentration of the conjugate was 1.8-fold higher than the control. Our study suggested that RcAAP1 as carrier was involved in the uptake and phloem translocation of L-Val-PCA, which could lay foundation for the utilization of amino acids and further development of vectorized agrochemicals.
Keywords: amino acid-pesticide conjugate; overexpression; phloem mobility; subcellular localization; uptake; vacuum agroinfiltration.
Copyright © 2023 Xiao, Hu, Hsiang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Arzola L., Chen J., Rattanaporn K., Maclean J. M., McDonald K. A. (2011). Transient co-expression of post-transcriptional gene silencing suppressors for increased in planta expression of a recombinant anthrax receptor fusion protein. Int. J. Mol. Sci. 12, 4975–4990. doi: 10.3390/ijms12084975 - DOI - PMC - PubMed
-
- Bush D. R. (1993). Proton-coupled sugar and amino acid transporters in plants. Annu. Rev. Plant Biol. 44, 513–542. doi: 10.1146/annurev.pp.44.060193.002501 - DOI
-
- Chollet J. F., Rocher F., Jousse C., Delétage-Grandon C., Bashiardes G., Bonnemain J. L. (2005). Acidic derivatives of the fungicide fenpiclonil: effect of adding a methyl group to the n-substituted chain on systemicity and fungicidal activity. Pest Manage. Sci. 61, 377–382. doi: 10.1002/ps.977 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

