Study on Commercially Available Membranes for Alkaline Direct Ethanol Fuel Cells
- PMID: 37332806
- PMCID: PMC10269243
- DOI: 10.1021/acsomega.3c01564
Study on Commercially Available Membranes for Alkaline Direct Ethanol Fuel Cells
Abstract
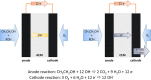
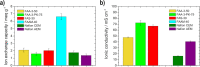
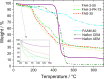
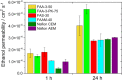
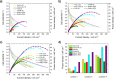
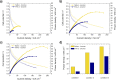
This study provides a comparison of different commercially available low-cost anion exchange membranes (AEMs), a microporous separator, a cation exchange membrane (CEM), and an anionic-treated CEM for their application in the liquid-feed alkaline direct ethanol fuel cell (ADEFC). Moreover, the effect on performance was evaluated taking two different modes of operation for the ADEFC, with AEM or CEM, into consideration. The membranes were compared with respect to their physical and chemical properties, such as thermal and chemical stability, ion-exchange capacity, ionic conductivity, and ethanol permeability. The influence of these factors on performance and resistance was determined by means of polarization curve and electrochemical impedance spectra (EIS) measurements in the ADEFC. In addition, the influence of two different commercial ionomers on the structure and transport properties of the catalyst layer and on the performance were analyzed with scanning electron microscopy, single cell tests, and EIS. The applicability barriers of the membranes were pointed out, and the ideal combinations of membrane and ionomer for the liquid-feed ADEFC achieved power densities of approximately 80 mW cm-2 at 80 °C.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Ferriday T. B.; Middleton P. H. Alkaline Fuel Cell Technology - A Review. Int. J. Hydrogen Energy 2021, 46, 18489–18510. 10.1016/j.ijhydene.2021.02.203. - DOI
-
- Hren M.; Božič M.; Fakin D.; Kleinschek K. S.; Gorgieva S. Alkaline Membrane Fuel Cells: Anion Exchange Membranes and Fuels. Sustain. Energy Fuels 2021, 5, 604–637. 10.1039/d0se01373k. - DOI
-
- Couture G.; Alaaeddine A.; Boschet F.; Ameduri B. Polymeric Materials as Anion-Exchange Membranes for Alkaline Fuel Cells. Prog. Polym. Sci. 2011, 36, 1521–1557. 10.1016/j.progpolymsci.2011.04.004. - DOI
-
- Yu E. H.; Wang X.; Krewer U.; Li L.; Scott K. Direct Oxidation Alkaline Fuel Cells: From Materials to Systems. Energy Environ. Sci. 2012, 5, 5668–5680. 10.1039/c2ee02552c. - DOI
-
- Zhao T. S.; Li Y. S.; Shen S. Y. Anion-Exchange Membrane Direct Ethanol Fuel Cells: Status and Perspective. Front. Energy Power Eng. China 2010, 4, 443–458. 10.1007/s11708-010-0127-5. - DOI
LinkOut - more resources
Full Text Sources

