Post-ischemic reorganization of sensory responses in cerebral cortex
- PMID: 37332854
- PMCID: PMC10272353
- DOI: 10.3389/fnins.2023.1151309
Post-ischemic reorganization of sensory responses in cerebral cortex
Abstract
Introduction: Sensorimotor integration is critical for generating skilled, volitional movements. While stroke tends to impact motor function, there are also often associated sensory deficits that contribute to overall behavioral deficits. Because many of the cortico-cortical projections participating in the generation of volitional movement either target or pass-through primary motor cortex (in rats, caudal forelimb area; CFA), any damage to CFA can lead to a subsequent disruption in information flow. As a result, the loss of sensory feedback is thought to contribute to motor dysfunction even when sensory areas are spared from injury. Previous research has suggested that the restoration of sensorimotor integration through reorganization or de novo neuronal connections is important for restoring function. Our goal was to determine if there was crosstalk between sensorimotor cortical areas with recovery from a primary motor cortex injury. First, we investigated if peripheral sensory stimulation would evoke responses in the rostral forelimb area (RFA), a rodent homologue to premotor cortex. We then sought to identify whether intracortical microstimulation-evoked activity in RFA would reciprocally modify the sensory response.
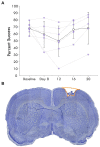
Methods: We used seven rats with an ischemic lesion of CFA. Four weeks after injury, the rats' forepaw was mechanically stimulated under anesthesia and neural activity was recorded in the cortex. In a subset of trials, a small intracortical stimulation pulse was delivered in RFA either individually or paired with peripheral sensory stimulation.
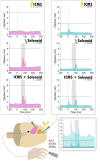
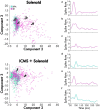
Results: Our results point to post-ischemic connectivity between premotor and sensory cortex that may be related to functional recovery. Premotor recruitment during the sensory response was seen with a peak in spiking within RFA after the peripheral solenoid stimulation despite the damage to CFA. Furthermore, stimulation in RFA modulated and disrupted the sensory response in sensory cortex.
Discussion: The presence of a sensory response in RFA and the sensitivity of S1 to modulation by intracortical stimulation provides additional evidence for functional connectivity between premotor and somatosensory cortex. The strength of the modulatory effect may be related to the extent of the injury and the subsequent reshaping of cortical connections in response to network disruption.
Keywords: activity; cerebral cortex; plasticity; somatosensory; stimulation.
Copyright © 2023 Hayley, Tuchek, Dalla, Borrell, Murphy, Nudo and Guggenmos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Update of
-
Post-Ischemic Reorganization of Sensory Responses in Cerebral Cortex.bioRxiv [Preprint]. 2023 Jan 20:2023.01.18.524583. doi: 10.1101/2023.01.18.524583. bioRxiv. 2023. Update in: Front Neurosci. 2023 Jun 02;17:1151309. doi: 10.3389/fnins.2023.1151309. PMID: 36711682 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

