TRP channels: Role in neurodegenerative diseases and therapeutic targets
- PMID: 37332910
- PMCID: PMC10272313
- DOI: 10.1016/j.heliyon.2023.e16910
TRP channels: Role in neurodegenerative diseases and therapeutic targets
Abstract
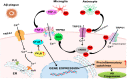
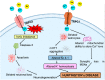
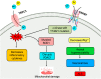
TRP (Transient receptor potential) channels are integral membrane proteins consisting of a superfamily of cation channels that allow permeability of both monovalent and divalent cations. TRP channels are subdivided into six subfamilies: TRPC, TRPV, TRPM, TRPP, TRPML, and TRPA, and are expressed in almost every cell and tissue. TRPs play an instrumental role in the regulation of various physiological processes. TRP channels are extensively represented in brain tissues and are present in both prokaryotes and eukaryotes, exhibiting responses to several mechanisms, including physical, chemical, and thermal stimuli. TRP channels are involved in the perturbation of Ca2+ homeostasis in intracellular calcium stores, both in neuronal and non-neuronal cells, and its discrepancy leads to several neuronal disorders such as Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), and Amyotrophic lateral sclerosis (ALS). TRPs participate in neurite outgrowth, receptor signaling, and excitotoxic cell death in the central nervous system. Understanding the mechanism of TRP channels in neurodegenerative diseases may extend to developing novel therapies. Thus, this review articulates TRP channels' physiological and pathological role in exploring new therapeutic interventions in neurodegenerative diseases.
Keywords: Alzheimer's disease; Amyotrophic lateral sclerosis; Ca2+ homeostasis; Huntington's disease; Parkinson's disease; TRP.
© 2023 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:One of the author Dr Syam Mohan is a member of Editorial board.
Figures

Similar articles
-
Targeting TRPs in neurodegenerative disorders.Curr Top Med Chem. 2013;13(3):322-34. doi: 10.2174/1568026611313030009. Curr Top Med Chem. 2013. PMID: 23432063 Review.
-
The role of transient receptor potential cation channels in Ca2+ signaling.Cold Spring Harb Perspect Biol. 2010 Oct;2(10):a003962. doi: 10.1101/cshperspect.a003962. Epub 2010 Sep 22. Cold Spring Harb Perspect Biol. 2010. PMID: 20861159 Free PMC article.
-
TRP channels in health and disease at a glance.J Cell Sci. 2021 Jul 1;134(13):jcs258372. doi: 10.1242/jcs.258372. Epub 2021 Jul 13. J Cell Sci. 2021. PMID: 34254641 Free PMC article.
-
TRP ion channels in the nervous system.Curr Opin Neurobiol. 2004 Jun;14(3):362-9. doi: 10.1016/j.conb.2004.05.003. Curr Opin Neurobiol. 2004. PMID: 15194117 Review.
-
TRP channels in disease.Subcell Biochem. 2007;45:253-71. doi: 10.1007/978-1-4020-6191-2_9. Subcell Biochem. 2007. PMID: 18193640 Review.
Cited by
-
Mutation of TRPML1 Channel and Pathogenesis of Neurodegeneration in Haimeria.Mol Neurobiol. 2024 Aug;61(8):4992-5001. doi: 10.1007/s12035-023-03874-y. Epub 2023 Dec 29. Mol Neurobiol. 2024. PMID: 38157120 Review.
-
TLR4 induced TRPM2 mediated neuropathic pain.Front Pharmacol. 2024 Sep 12;15:1472771. doi: 10.3389/fphar.2024.1472771. eCollection 2024. Front Pharmacol. 2024. PMID: 39329114 Free PMC article. Review.
-
Dysregulation of Ion Channels and Transporters and Blood-Brain Barrier Dysfunction in Alzheimer's Disease and Vascular Dementia.Aging Dis. 2024 Aug 1;15(4):1748-1770. doi: 10.14336/AD.2023.1201. Aging Dis. 2024. PMID: 38300642 Free PMC article. Review.
-
Epidural injection of varying doses of capsaicin alleviates inflammatory pain in rats via the TLR4/AKT/NF-κB pathway.Inflammopharmacology. 2025 Jan;33(1):257-267. doi: 10.1007/s10787-024-01617-6. Epub 2024 Dec 17. Inflammopharmacology. 2025. PMID: 39690361 Free PMC article.
-
Oxidative stress and ion channels in neurodegenerative diseases.Front Physiol. 2024 Jan 29;15:1320086. doi: 10.3389/fphys.2024.1320086. eCollection 2024. Front Physiol. 2024. PMID: 38348223 Free PMC article. Review.
References
-
- Montell C. The TRP superfamily of cation channels. Sci. STKE. 2005;22:re3. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

