Effect of vaccine dosing intervals on Omicron surrogate neutralization after three doses of BNT162b2
- PMID: 37332982
- PMCID: PMC10263225
- DOI: 10.1016/j.heliyon.2023.e17259
Effect of vaccine dosing intervals on Omicron surrogate neutralization after three doses of BNT162b2
Abstract
Background: Increasing the interval between the first and second SARS-CoV-2 vaccine doses enhances vaccine immunogenicity, however the optimal timing of the third vaccine is unknown. In this study, we investigated how the time interval between the first and second (V1-V2), or second and third (V2-V3) doses affects immunogenicity after three doses of the BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine.
Methods: This is an observational cohort consisting of 360 participants enrolled in the COVID-19 Occupational Risks, Seroprevalence, and Immunity among Paramedics in Canada (CORSIP) study. Immune responses to BA.1 and other variants were measured from serum using an ACE2 competitive binding assay for surrogate SARS-CoV-2 neutralization. We fit a multiple linear regression model to estimate the independent association between both the V1-V2 and V2-V3 intervals and serum SARS-CoV-2 neutralization, while adjusting for age, sex, and the V3-to-blood collection interval. We examined vaccine dosing intervals as continuous variables and categorized them into quartiles.
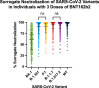
Results: The mean age was 40 years, 45% were female sex (at birth), and the median BA.1 surrogate neutralization was 61% (IQR 38-77%). The multivariate analysis indicated that longer V1-V2 (β = 0.1292, 95% CI: 0.04807-0.2104) and V2-V3 (β = 0.2653, 95% CI: 0.2291-0.3015) intervals were associated with increased surrogate neutralization of BA.1. These results were consistent when examining responses against Spike from other SARS-CoV-2 strains. When categorized into V2-V3 quartiles, the first (56-231 days), and second (231-266 days) quartiles demonstrated decreased BA.1 surrogate neutralization compared to the longest V2-V3 quartile (282-329 days). There was no significant difference in surrogate neutralization between the long (266-282 days) and longest (282-329 days) V2-V3 intervals.
Conclusion: Longer intervals between first, second and third doses are independently associated with increased immunogenicity for all tested SARS-CoV-2 strains. Increasing the intervals between the second and third vaccine doses up to 8.9 months provided additive benefits increasing the immunogenicity of BNT162b2 vaccine schedules.
Keywords: COVID; Immunization; SARS-CoV-2; Surrogate neutralization; Vaccine interval.
© 2023 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Brian Grunau reports financial support was provided by Government of Canada. Brian Grunau reports financial support was provided by Michael Smith Foundation for Health Research. Mohammad E. Karim reports financial support was provided by Michael Smith Foundation for Health Research. Michael Asamoah-Boaheng reports financial support was provided by Michael Smith Foundation for Health Research. Martin Prusinkiewicz reports financial support was provided by Canadian Institutes of Health Research.
Figures

References
-
- Mohapatra R.K., Kandi V., Sarangi A.K., Verma S., Tuli H.S., Chakraborty S., Chakraborty C., Dhama K. The recently emerged BA.4 and BA.5 lineages of Omicron and their global health concerns amid the ongoing wave of COVID-19 pandemic – correspondence. Int. J. Surg. 2022;103 doi: 10.1016/j.ijsu.2022.106698. - DOI - PMC - PubMed
-
- Payne R.P., Longet S., Austin J.A., Skelly D.T., Dejnirattisai W., Adele S., Meardon N., Faustini S., Al-Taei S., Moore S.C., Tipton T., Hering L.M., Angyal A., Brown R., Nicols A.R., Gillson N., Dobson S.L., Amini A., Supasa P., Cross A., Bridges-Webb A., Reyes L.S., Linder A., Sandhar G., Kilby J.A., Tyerman J.K., Altmann T., Hornsby H., Whitham R., Phillips E., Malone T., Hargreaves A., Shields A., Saei A., Foulkes S., Stafford L., Johnson S., Wootton D.G., Conlon C.P., Jeffery K., Matthews P.C., Frater J., Deeks A.S., Pollard A.J., Brown A., Rowland-Jones S.L., Mongkolsapaya J., Barnes E., Hopkins S., Hall V., Dold C., Duncan C.J.A., Richter A., Carroll M., Screaton G., de Silva T.I., Turtle L., Klenerman P., Dunachie S., Abuelgasim H., Adland E., Adlou S., Akther H.D., Alhussni A., Ali M., Ansari M.A., v Arancibia-Cárcamo C., Bayley M., Brown H., Chalk J., Chand M., Chawla A., Chinnakannan S., Cutteridge J., de Lara C., Denly L., Diffey B., Dimitriadis S., Drake T.M., Donnison T., Dupont M., Eyre D., Fairman A., Gardiner S., Gilbert-Jarmillo J., Goulder P., Hackstein C.-P., Hambleton S., Haniffa M., Haworth J., Holmes J., Horner E., Jämsén A., Johnson S., Jones C., Kasanyinga M., Kelly S., Kirk R., Knight M.L., Lawrie A., Lee L., Lett L., Lillie K., Lim N., Mehta H., Mentzer A.J., O'Donnell D., Ogbe A., Pace M., Payne B.A.I., Platt G., Poolan S., Provine N., Ramamurthy N., Robinson N., Romaniuk L., Rongkard P., Sampson O.L., Simmons B., Spegarova J.S., Stephenson E., Subramaniam K., Thaventhiran J., Thomas S., Travis S., Tucker S., Turton H., Watson A., Watson L., Weeks E., Wilson R., Wood S., Wright R., Xiao H., Zawia A.A.T. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184:5699–5714.e11. doi: 10.1016/j.cell.2021.10.011. - DOI - PMC - PubMed
-
- Grunau B., Asamoah-Boaheng M., Lavoie P.M., Karim M.E., Kirkham T.L., Demers P.A., Barakauskas V., Marquez A.C., Jassem A.N., O'Brien S.F., Drews S.J., Haig S., Cheskes S., Goldfarb D.M. Clinical Infectious Diseases; 2021. A Higher Antibody Response Is Generated with a 6- to 7-Week (Vs Standard) Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Dosing Interval; p. ciab938. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

