Fibroblast Activation Protein Expression in Sarcomas
- PMID: 37333052
- PMCID: PMC10275689
- DOI: 10.1155/2023/2480493
Fibroblast Activation Protein Expression in Sarcomas
Abstract
Objectives: Fibroblast activation protein alpha (FAP) is highly expressed by cancer-associated fibroblasts in multiple epithelial cancers. The aim of this study was to characterize FAP expression in sarcomas to explore its potential utility as a diagnostic and therapeutic target and prognostic biomarker in sarcomas.
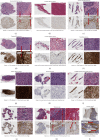
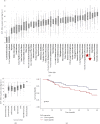
Methods: Available tissue samples from patients with bone or soft tissue tumors were identified at the University of California, Los Angeles. FAP expression was evaluated via immunohistochemistry (IHC) in tumor samples (n = 63), adjacent normal tissues (n = 30), and positive controls (n = 2) using semiquantitative systems for intensity (0 = negative; 1 = weak; 2 = moderate; and 3 = strong) and density (none, <25%, 25-75%; >75%) in stromal and tumor/nonstromal cells and using a qualitative overall score (not detected, low, medium, and high). Additionally, RNA sequencing data in publicly available databases were utilized to compare FAP expression in samples (n = 10,626) from various cancer types and evaluate the association between FAP expression and overall survival (OS) in sarcoma (n = 168).
Results: The majority of tumor samples had FAP IHC intensity scores ≥2 and density scores ≥25% for stromal cells (77.7%) and tumor cells (50.7%). All desmoid fibromatosis, myxofibrosarcoma, solitary fibrous tumor, and undifferentiated pleomorphic sarcoma samples had medium or high FAP overall scores. Sarcomas were among cancer types with the highest mean FAP expression by RNA sequencing. There was no significant difference in OS in patients with sarcoma with low versus high FAP expression.
Conclusion: The majority of the sarcoma samples showed FAP expression by both stromal and tumor/nonstromal cells. Further investigation of FAP as a potential diagnostic and therapeutic target in sarcomas is warranted.
Copyright © 2023 Jacquelyn N. Crane et al.
Conflict of interest statement
F.C.E. is on the Scientific Advisory Board of Certis Oncology. T.G.G. has consulting and equity agreements with Auron Therapeutics, Boundless Bio, Coherus BioSciences, and Trethera Corporation. The authors declare that they have no conflicts of interest.
Figures

References
-
- Fletcher C. D. M. B. J., Hogendoorn P. C. W., Mertens F. World Health Organization Classification of Tumours of Soft Tissue and Bone . 4. Lyon, France: International Agency for Research on Cancer Press; 2013.
-
- Federman N. V. D. E., Bernthal N. Lanzkoswky’s Manual of Pediatric Hematology and Oncology . Amsterdam, Netherlands: Elsevier; 2016. Malignant bone tumors; pp. 523–543.
-
- Fein Levy C. W. L. Lanzkoswky’s Manual of Pediatric Hematology and Oncology . Amsterdam, Netherlands: Elsevier; 2016. Rhabdomyosarcoma andf other soft-tissue sarcomas; pp. 505–523.
-
- National Cancer Institute. Surveillance, Epidemiology and End Results Program . National Cancer Institute; 2019. https://seer.cancer.gov/statfacts/
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

