This is a preprint.
The full spectrum of OCT1 (SLC22A1) mutations bridges transporter biophysics to drug pharmacogenomics
- PMID: 37333090
- PMCID: PMC10274788
- DOI: 10.1101/2023.06.06.543963
The full spectrum of OCT1 (SLC22A1) mutations bridges transporter biophysics to drug pharmacogenomics
Update in
-
The full spectrum of SLC22 OCT1 mutations illuminates the bridge between drug transporter biophysics and pharmacogenomics.Mol Cell. 2024 May 16;84(10):1932-1947.e10. doi: 10.1016/j.molcel.2024.04.008. Epub 2024 May 3. Mol Cell. 2024. PMID: 38703769 Free PMC article.
Abstract
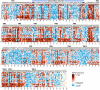
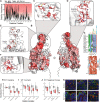
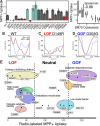
Membrane transporters play a fundamental role in the tissue distribution of endogenous compounds and xenobiotics and are major determinants of efficacy and side effects profiles. Polymorphisms within these drug transporters result in inter-individual variation in drug response, with some patients not responding to the recommended dosage of drug whereas others experience catastrophic side effects. For example, variants within the major hepatic Human organic cation transporter OCT1 (SLC22A1) can change endogenous organic cations and many prescription drug levels. To understand how variants mechanistically impact drug uptake, we systematically study how all known and possible single missense and single amino acid deletion variants impact expression and substrate uptake of OCT1. We find that human variants primarily disrupt function via folding rather than substrate uptake. Our study revealed that the major determinants of folding reside in the first 300 amino acids, including the first 6 transmembrane domains and the extracellular domain (ECD) with a stabilizing and highly conserved stabilizing helical motif making key interactions between the ECD and transmembrane domains. Using the functional data combined with computational approaches, we determine and validate a structure-function model of OCT1s conformational ensemble without experimental structures. Using this model and molecular dynamic simulations of key mutants, we determine biophysical mechanisms for how specific human variants alter transport phenotypes. We identify differences in frequencies of reduced function alleles across populations with East Asians vs European populations having the lowest and highest frequency of reduced function variants, respectively. Mining human population databases reveals that reduced function alleles of OCT1 identified in this study associate significantly with high LDL cholesterol levels. Our general approach broadly applied could transform the landscape of precision medicine by producing a mechanistic basis for understanding the effects of human mutations on disease and drug response.
Conflict of interest statement
Competing interests The authors declare they have no competing interests.
Figures



Similar articles
-
The full spectrum of SLC22 OCT1 mutations illuminates the bridge between drug transporter biophysics and pharmacogenomics.Mol Cell. 2024 May 16;84(10):1932-1947.e10. doi: 10.1016/j.molcel.2024.04.008. Epub 2024 May 3. Mol Cell. 2024. PMID: 38703769 Free PMC article.
-
Global genetic analyses reveal strong inter-ethnic variability in the loss of activity of the organic cation transporter OCT1.Genome Med. 2015 Jun 18;7(1):56. doi: 10.1186/s13073-015-0172-0. eCollection 2015. Genome Med. 2015. PMID: 26157489 Free PMC article.
-
Amino acids in transmembrane helix 1 confer major functional differences between human and mouse orthologs of the polyspecific membrane transporter OCT1.J Biol Chem. 2022 Jun;298(6):101974. doi: 10.1016/j.jbc.2022.101974. Epub 2022 Apr 22. J Biol Chem. 2022. PMID: 35469921 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
The human organic cation transporter OCT1 and its role as a target for drug responses.Drug Metab Rev. 2019 Nov;51(4):389-407. doi: 10.1080/03602532.2019.1670204. Epub 2019 Sep 28. Drug Metab Rev. 2019. PMID: 31564168 Review.
References
-
- Bushnell B. (2014) ‘BBTools software package’.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
