This is a preprint.
Longitudinal host transcriptional responses to SARS-CoV-2 infection in adults with extremely high viral load
- PMID: 37333115
- PMCID: PMC10274945
- DOI: 10.21203/rs.3.rs-2978272/v1
Longitudinal host transcriptional responses to SARS-CoV-2 infection in adults with extremely high viral load
Update in
-
Longitudinal host transcriptional responses to SARS-CoV-2 infection in adults with extremely high viral load.PLoS One. 2025 Jan 16;20(1):e0317033. doi: 10.1371/journal.pone.0317033. eCollection 2025. PLoS One. 2025. PMID: 39820858 Free PMC article.
Abstract
Current understanding of viral dynamics of SARS-CoV-2 and host responses driving the pathogenic mechanisms in COVID-19 is rapidly evolving. Here, we conducted a longitudinal study to investigate gene expression patterns during acute SARS-CoV-2 illness. Cases included SARS-CoV-2 infected individuals with extremely high viral loads early in their illness, individuals having low SARS-CoV-2 viral loads early in their infection, and individuals testing negative for SARS-CoV-2. We could identify widespread transcriptional host responses to SARS-CoV-2 infection that were initially most strongly manifested in patients with extremely high initial viral loads, then attenuating within the patient over time as viral loads decreased. Genes correlated with SARS-CoV-2 viral load over time were similarly differentially expressed across independent datasets of SARS-CoV-2 infected lung and upper airway cells, from both in vitro systems and patient samples. We also generated expression data on the human nose organoid model during SARS-CoV-2 infection. The human nose organoid-generated host transcriptional response captured many aspects of responses observed in the above patient samples, while suggesting the existence of distinct host responses to SARS-CoV-2 depending on the cellular context, involving both epithelial and cellular immune responses. Our findings provide a catalog of SARS-CoV-2 host response genes changing over time.
Conflict of interest statement
Conflict of Interest statement: The authors declare no competing interests.
Figures

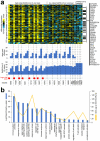

References
-
- He X. et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 26, 672–675 (2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

