This is a preprint.
Glucose Hypometabolism Prompts RAN Translation and Exacerbates C9orf72-related ALS/FTD Phenotypes
- PMID: 37333144
- PMCID: PMC10274806
- DOI: 10.1101/2023.06.07.544100
Glucose Hypometabolism Prompts RAN Translation and Exacerbates C9orf72-related ALS/FTD Phenotypes
Update in
-
Glucose hypometabolism prompts RAN translation and exacerbates C9orf72-related ALS/FTD phenotypes.EMBO Rep. 2024 May;25(5):2479-2510. doi: 10.1038/s44319-024-00140-7. Epub 2024 Apr 29. EMBO Rep. 2024. PMID: 38684907 Free PMC article.
Abstract
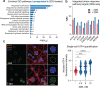
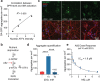
The most prevalent genetic cause of both amyotrophic lateral sclerosis and frontotemporal dementia is a (GGGGCC)n nucleotide repeat expansion (NRE) occurring in the first intron of the C9orf72 gene (C9). Brain glucose hypometabolism is consistently observed in C9-NRE carriers, even at pre-symptomatic stages, although its potential role in disease pathogenesis is unknown. Here, we identified alterations in glucose metabolic pathways and ATP levels in the brain of asymptomatic C9-BAC mice. We found that, through activation of the GCN2 kinase, glucose hypometabolism drives the production of dipeptide repeat proteins (DPRs), impairs the survival of C9 patient-derived neurons, and triggers motor dysfunction in C9-BAC mice. We also found that one of the arginine-rich DPRs (PR) can directly contribute to glucose metabolism and metabolic stress. These findings provide a mechanistic link between energy imbalances and C9-ALS/FTD pathogenesis and support a feedforward loop model that opens several opportunities for therapeutic intervention.
Keywords: ALS; C9orf72; Dipeptide Repeat Proteins; FTD; Glucose Hypometabolism; RAN Translation.
Conflict of interest statement
Disclosure and Competing Interests Statement The authors declare no competing financial interests.
Figures





References
-
- Ash Peter EA, Bieniek Kevin F, Gendron Tania F, Caulfield T, Lin W-L, DeJesus-Hernandez M, van Blitterswijk Marka M, Jansen-West K, Paul Joseph W, Rademakers R et al. (2013) Unconventional Translation of C9ORF72 GGGGCC Expansion Generates Insoluble Polypeptides Specific to c9FTD/ALS. Neuron 77: 639–646 - PMC - PubMed
-
- Beghi E, Pupillo E, Bonito V, Buzzi P, Caponnetto C, Chio A, Corbo M, Giannini F, Inghilleri M, Bella VL et al. (2013) Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for ALS. Amyotroph Lateral Scler Frontotemporal Degener 14: 397–405 - PubMed
-
- Chighine A, Locci E, Nioi M, d’Aloja E (2021) Looking for Post-Mortem Metabolomic Standardization: Waiting for Godot-The Importance of Post-Mortem Interval in Forensic Metabolomics. Chem Res Toxicol 34: 1946–1947 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
