This is a preprint.
Genetically engineering endothelial niche in human kidney organoids enables multilineage maturation, vascularization and de novo cell types
- PMID: 37333155
- PMCID: PMC10274893
- DOI: 10.1101/2023.05.30.542848
Genetically engineering endothelial niche in human kidney organoids enables multilineage maturation, vascularization and de novo cell types
Update in
-
A genetically inducible endothelial niche enables vascularization of human kidney organoids with multilineage maturation and emergence of renin expressing cells.Kidney Int. 2024 Dec;106(6):1086-1100. doi: 10.1016/j.kint.2024.05.026. Epub 2024 Jun 18. Kidney Int. 2024. PMID: 38901605 Free PMC article.
Abstract
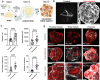
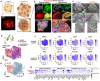
Vascularization plays a critical role in organ maturation and cell type development. Drug discovery, organ mimicry, and ultimately transplantation in a clinical setting thereby hinges on achieving robust vascularization of in vitro engineered organs. Here, focusing on human kidney organoids, we overcome this hurdle by combining an inducible ETS translocation variant 2 (ETV2) human induced pluripotent stem cell (iPSC) line, which directs endothelial fate, with a non-transgenic iPSC line in suspension organoid culture. The resulting human kidney organoids show extensive vascularization by endothelial cells with an identity most closely related to endogenous kidney endothelia. Vascularized organoids also show increased maturation of nephron structures including more mature podocytes with improved marker expression, foot process interdigitation, an associated fenestrated endothelium, and the presence of renin+ cells. The creation of an engineered vascular niche capable of improving kidney organoid maturation and cell type complexity is a significant step forward in the path to clinical translation. Furthermore, this approach is orthogonal to native tissue differentiation paths, hence readily adaptable to other organoid systems and thus has the potential for a broad impact on basic and translational organoid studies.
Keywords: Genetic engineering; endothelial; organoids; podocytes; renin; scRNAseq.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
