This is a preprint.
A Spatiotemporal Compartmentalization of Glucose Metabolism Guides Mammalian Gastrulation Progression
- PMID: 37333168
- PMCID: PMC10274656
- DOI: 10.1101/2023.06.06.543780
A Spatiotemporal Compartmentalization of Glucose Metabolism Guides Mammalian Gastrulation Progression
Abstract
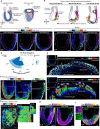
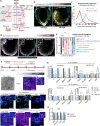
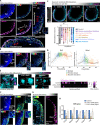
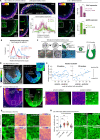
Gastrulation is considered the sine qua non of embryogenesis, establishing a multidimensional structure and the spatial coordinates upon which all later developmental events transpire. At this time, the embryo adopts a heavy reliance on glucose metabolism to support rapidly accelerating changes in morphology, proliferation, and differentiation. However, it is currently unknown how this conserved metabolic shift maps onto the three-dimensional landscape of the growing embryo and whether it is spatially linked to the orchestrated cellular and molecular processes necessary for gastrulation. Here we identify that glucose is utilised during mouse gastrulation via distinct metabolic pathways to instruct local and global embryonic morphogenesis, in a cell type and stage-specific manner. Through detailed mechanistic studies and quantitative live imaging of mouse embryos, in parallel with tractable in vitro stem cell differentiation models and embryo-derived tissue explants, we discover that cell fate acquisition and the epithelial-to-mesenchymal transition (EMT) relies on the Hexosamine Biosynthetic Pathway (HBP) branch of glucose metabolism, while newly-formed mesoderm requires glycolysis for correct migration and lateral expansion. This regional and tissue-specific difference in glucose metabolism is coordinated with Fibroblast Growth Factor (FGF) activity, demonstrating that reciprocal crosstalk between metabolism and growth factor signalling is a prerequisite for gastrulation progression. We expect these studies to provide important insights into the function of metabolism in other developmental contexts and may help uncover mechanisms that underpin embryonic lethality, cancer, and congenital disease.
Conflict of interest statement
Conflict of interest statement The authors declare no conflict of interest.
Figures


References
-
- Arnold S.J., and Robertson E.J. (2009). Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol 10, 91–103. - PubMed
-
- Artym V.V., Yamada K.M., and Mueller S.C. (2009). ECM degradation assays for analyzing local cell invasion. Methods Mol Biol 522, 211–219. - PubMed
-
- Barban S., and Schulze H.O. (1961). The effects of 2-deoxyglucose on the growth and metabolism of cultured human cells. J Biol Chem 236, 1887–1890. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
