This is a preprint.
Recording morphogen signals reveals origins of gastruloid symmetry breaking
- PMID: 37333235
- PMCID: PMC10274695
- DOI: 10.1101/2023.06.02.543474
Recording morphogen signals reveals origins of gastruloid symmetry breaking
Update in
-
Recording morphogen signals reveals mechanisms underlying gastruloid symmetry breaking.Nat Cell Biol. 2024 Nov;26(11):1832-1844. doi: 10.1038/s41556-024-01521-9. Epub 2024 Oct 2. Nat Cell Biol. 2024. PMID: 39358450 Free PMC article.
Abstract
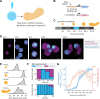
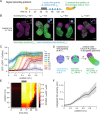
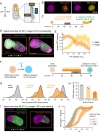
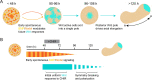
When cultured in three dimensional spheroids, mammalian stem cells can reproducibly self-organize a single anterior-posterior axis and sequentially differentiate into structures resembling the primitive streak and tailbud. Whereas the embryo's body axes are instructed by spatially patterned extra-embryonic cues, it is unknown how these stem cell gastruloids break symmetry to reproducibly define a single anterior-posterior (A-P) axis. Here, we use synthetic gene circuits to trace how early intracellular signals predict cells' future anterior-posterior position in the gastruloid. We show that Wnt signaling evolves from a homogeneous state to a polarized state, and identify a critical 6-hour time period when single-cell Wnt activity predicts future cellular position, prior to the appearance of polarized signaling patterns or morphology. Single-cell RNA sequencing and live-imaging reveal that early Wnt-high and Wnt-low cells contribute to distinct cell types and suggest that axial symmetry breaking is driven by sorting rearrangements involving differential cell adhesion. We further extend our approach to other canonical embryonic signaling pathways, revealing that even earlier heterogeneity in TGFβ signaling predicts A-P position and modulates Wnt signaling during the critical time period. Our study reveals a sequence of dynamic cellular processes that transform a uniform cell aggregate into a polarized structure and demonstrates that a morphological axis can emerge out of signaling heterogeneity and cell movements even in the absence of exogenous patterning cues.
Keywords: Gastruloid; cell signaling; self-organization; symmetry breaking.
Conflict of interest statement
Competing Interests J.E.T. is a scientific advisor for Prolific Machines and Nereid Therapeutics. B.A. is an advisory board member for Arbor Biotechnologies and Tessera Therapeutics and holds equity in Celsius Therapeutics. H.M.M. is a cofounder and scientific advisor for C16 Biosciences. The remaining authors declare no conflicts of interest.
Figures



References
-
- Spemann H., and Mangold H. (1924). über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Archiv f mikr Anat u Entwicklungsmechanik 100, 599–638. 10.1007/BF02108133. - DOI
-
- Kondo S., and Miura T. (2010). Reaction-Diffusion Model as a Framework for Understanding Biological Pattern Formation. Science 329, 1616–1620. 10/bhd5cf. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
