This is a preprint.
SMYD5 is a regulator of the mild hypothermia response
- PMID: 37333301
- PMCID: PMC10274674
- DOI: 10.1101/2023.05.11.540170
SMYD5 is a regulator of the mild hypothermia response
Update in
-
SMYD5 is a regulator of the mild hypothermia response.Cell Rep. 2024 Aug 27;43(8):114554. doi: 10.1016/j.celrep.2024.114554. Epub 2024 Jul 30. Cell Rep. 2024. PMID: 39083378 Free PMC article.
Abstract
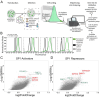
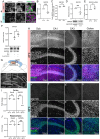
The mild hypothermia response (MHR) maintains organismal homeostasis during cold exposure and is thought to be critical for the neuroprotection documented with therapeutic hypothermia. To date, little is known about the transcriptional regulation of the MHR. We utilize a forward CRISPR-Cas9 mutagenesis screen to identify the histone lysine methyltransferase SMYD5 as a regulator of the MHR. SMYD5 represses the key MHR gene SP1 at euthermia. This repression correlates with temperature-dependent levels of H3K36me3 at the SP1-locus and globally, indicating that the mammalian MHR is regulated at the level of histone modifications. We have identified 37 additional SMYD5 regulated temperature-dependent genes, suggesting a broader MHR-related role for SMYD5. Our study provides an example of how histone modifications integrate environmental cues into the genetic circuitry of mammalian cells and provides insights that may yield therapeutic avenues for neuroprotection after catastrophic events.
Conflict of interest statement
Competing interests: HTB is a consultant for Mahzi Therapeutics and founder of Kaldur Therapeutics. SR and HTB have a European patent application (#23167505.9) on a therapeutic strategy to activate the MHR. Other authors declare that they have no competing interests.
Figures



References
-
- Gluckman P.D., Wyatt J.S., Azzopardi D., Ballard R., Edwards A.D., Ferriero D.M., Polin R.A., Robertson C.M., Thoresen M., Whitelaw A., et al. (2005). Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet 365, 663–670. 10.1016/S0140-6736(05)17946-X. - DOI - PubMed
-
- Dankiewicz J., Cronberg T., Lilja G., Jakobsen J.C., Levin H., Ullén S., Rylander C., Wise M.P., Oddo M., Cariou A., et al. (2021). Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 384, 2283–2294. 10.1056/NEJMOA2100591/SUPPL_FILE/NEJMOA2100591_DATA-SHARING.PDF. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
