This is a preprint.
A spatial map of hepatic mitochondria uncovers functional heterogeneity shaped by nutrient-sensing signaling
- PMID: 37333328
- PMCID: PMC10274915
- DOI: 10.1101/2023.04.13.536717
A spatial map of hepatic mitochondria uncovers functional heterogeneity shaped by nutrient-sensing signaling
Update in
-
A spatial map of hepatic mitochondria uncovers functional heterogeneity shaped by nutrient-sensing signaling.Nat Commun. 2024 Feb 28;15(1):1799. doi: 10.1038/s41467-024-45751-9. Nat Commun. 2024. PMID: 38418824 Free PMC article.
Abstract
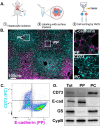
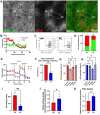
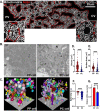
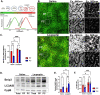
In the liver, mitochondria are exposed to different concentrations of nutrients due to their spatial positioning across the periportal (PP) and pericentral (PC) axis. How these mitochondria sense and integrate these signals to respond and maintain homeostasis is not known. Here, we combined intravital microscopy, spatial proteomics, and functional assessment to investigate mitochondrial heterogeneity in the context of liver zonation. We found that PP and PC mitochondria are morphologically and functionally distinct; beta-oxidation was elevated in PP regions, while lipid synthesis was predominant in the PC mitochondria. In addition, comparative phosphoproteomics revealed spatially distinct patterns of mitochondrial composition and potential regulation via phosphorylation. Acute pharmacological modulation of nutrient sensing through AMPK and mTOR shifted mitochondrial phenotypes in the PP and PC regions, linking nutrient gradients across the lobule and mitochondrial heterogeneity. This study highlights the role of protein phosphorylation in mitochondrial structure, function, and overall homeostasis in hepatic metabolic zonation. These findings have important implications for liver physiology and disease.
Conflict of interest statement
The authors have no conflicts to report.
Figures


References
-
- Picard M. & Shirihai O. S. Mitochondrial signal transduction. Cell Metab 34, 1620–1653 (2022). https://doi.org: 10.1016/j.cmet.2022.10.008 - DOI - PMC - PubMed
-
- Glancy B. Visualizing Mitochondrial Form and Function within the Cell. Trends Mol Med 26, 58–70 (2020). https://doi.org: 10.1016/j.molmed.2019.09.009 - DOI - PMC - PubMed
-
- Hoppins S. The regulation of mitochondrial dynamics. Curr Opin Cell Biol 29, 46–52 (2014). https://doi.org: 10.1016/j.ceb.2014.03.005 - DOI - PubMed
-
- Friedman J. R. & Nunnari J. Mitochondrial form and function. Nature 505, 335–343 (2014). https://doi.org: 10.1038/nature12985 - DOI - PMC - PubMed
-
- Westermann B. Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys Acta 1817, 1833–1838 (2012). https://doi.org: 10.1016/j.bbabio.2012.02.033 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
